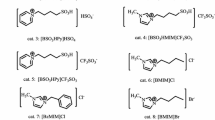
A series of novel pyrazolo[1,2-b]phthalazine-2-carboxylate derivatives has been synthesized via one-pot four-component reaction of aromatic aldehyde, ethyl or methyl acetoacetate, hydrazine hydrate, and phthalic anhydride in the presence of acidic ionic liquids such as 3-methyl-1-sulfo-1H-imidazol-3-ium chloride, 1,3-disulfo-1H-imidazol-3-ium chloride, and triethyl(sulfo)ammonium chloride as the catalyst. This method involves particular advantages such as environmentally benign catalysts, high yields of product, reusability of ionic liquids with high activity, short reaction times, no hazardous solvent, and easy workup.

Similar content being viewed by others
References
Sayyafi, M.; Seyyedhamzeh, M.; Khavasi, H. R.; Bazgir, A. Tetrahedron 2008, 64, 2375.
Zhang, L.; Guan, L. P.; Sun, X.-Y.; Wei, C.-X.; Chai, K.-Y.; Quan, Z. S. Chem. Biol. Drug Des. 2009, 73, 313.
Ryu, C.-K.; Park, R.-E.; Ma, M.-Y.; Nho, J.-H. Bioorg. Med. Chem. Lett. 2007, 17, 2577.
Li, J.; Zhao, Y.-F.; Yuan, X.-Y.; Xu, J.-X.; Gong, P. Molecules 2006, 11, 574.
Liu, D.-C.; Gong, G.-H.; Wei, C.-X.; Jin, X.-J.; Quan, Z.-S. Bioorg. Med. Chem. Lett. 2016, 26, 1576.
Nomoto, Y.; Obase, H.; Takai, H.; Teranishi, M.; Nakamura, J.; Kubo, K. Chem. Pharm. Bull. 1990, 38, 2179.
Terrett, N. K.; Bell, A. S.; Brown, D.; Ellis, P. Bioorg. Med. Chem. Lett. 1996, 6, 1819.
Singh, S. K.; Reddy, P. G.; Rao, K. S.; Lohray, B. B.; Misra, P.; Rajjak, S. A.; Rao, Y. K.; Venkateswarlu, A. Bioorg. Med. Chem. Lett. 2004, 14, 499.
Genin, M. J.; Biles, C.; Keiser, B. J.; Poppe, S. M.; Swaney, S. M.; Tarpley, W. G.; Yagi, Y.; Romero, D. L. J. Med. Chem. 2000, 43, 1034.
Arlan, F. M.; Khalafy, J.; Maleki, R. Chem. Heterocycl. Compd. 2018, 54, 51. [Khim. Geterotsikl. Soedin. 2018, 54, 51.]
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem.–Eur. J. 2000, 6, 3321.
Nefzi, A.; Ostresh, J. M.; Houghten, R. A. Chem. Rev. 1997, 97, 449.
Thompson, L. A. Curr. Opin. Chem. Biol. 2000, 4, 324.
Dömling, A. Curr. Opin. Chem. Biol. 2002, 6, 306.
Mizushima, E.; Hayashi, T.; Tanaka, M. Green Chem. 2001, 3, 76.
Yadav, J. S.; Reddy, B. V. S.; Baishya, G.; Reddy, K. V.; Narsaiah, A. V. Tetrahedron 2005, 61, 9541.
Picquet, M.; Stutzmann, S.; Tkatchenko, I.; Tommasi, I.; Zimmermann, J.; Wasserscheid, P. Green Chem. 2003, 5, 153.
Earle, M. J.; Katdare, S. P.; Seddon, K. R. Org. Lett. 2004, 6, 707.
Earle, M. J.; McCormac, P. B.; Seddon, K. R. Green Chem. 1999, 1, 23.
Chauvin, Y.; Mussmann, L.; Olivier, H. Angew. Chem., Int. Ed. Engl. 1996, 34, 2698.
Wilkes, J. S. Green Chem. 2002, 4, 73.
Ghahremanzadeh, R.; Shakibaei, G. I.; Bazgir, A. Synlett 2008, 1129.
Nabid, M. R.; Rezaei, S. J.; Ghahremanzadeh, R.; Bazgir, A. Ultrason. Sonochem. 2010, 17, 159.
Khurana, J. M.; Magoo, D. Tetrahedron Lett. 2009, 50, 7300.
Wang, H.-J.; Zhang, X.-N.; Zhang, Z.-H. Monatsh. Chem. 2010, 141, 425.
Shaterian, H. R.; Ghashang, M.; Feyzi, M. Appl. Catal., A 2008, 345, 128.
Saghanezhad, S. J.; Sayyahi, S. Res. Chem. Intermed. 2017, 43, 2491.
Goli-Jolodar, O.; Shirini, F. Theor. Exp. Chem. 2017, 52, 349.
Atar, A. B.; Lee, S. D.; Cho, B. G.; Cho, D. W.; Jeong, Y. T. Res. Chem. Intermed. 2016, 42, 1707.
Maleki, B.; Sedigh Ashrafi, S. J. Mex. Chem. Soc. 2014, 58, 159.
Veisi, H.; Manesh, A. A.; Khankhani, N.; Ghorbani-Vaghei, R. RSC Adv. 2014, 4, 25057.
Shirini, F.; Langarudi, M. S. N.; Goli-Jolodar, O. Dyes Pigm. 2015, 123, 186.
Pouramiri, B.; Tavakolinejad Kermani, E. Tetrahedron Lett. 2016, 57, 1006.
Pouramiri, B.; Tavakolinejad Kermani, E. J. Iran. Chem. Soc. 2016, 13, 1011.
Zahedifar, M.; Shojaei, R.; Sheibani, H. Res. Chem. Intermed. 2018, 44, 873.
Zahedifar, M.; Mohammadi, P.; Sheibani, H. Lett. Org. Chem. 2017, 14, 315.
Zolfigol, M. A.; Khazaei, A.; Moosavi-Zare, A. R.; Zare, A. J. Iran. Chem. Soc. 2010, 7, 646.
Zare, A.; Moosavi-Zare, A. R.; Merajoddin, M.; Zolfigol, M. A.; Hekmat-Zadeh, T.; Hasaninejad, A.; Khazaei, A.; Mokhlesi, M.; Khakyzadeh, V.; Derakhshan-Panah, F.; Beyzavi, M. H.; Rostami, E.; Arghoon, A.; Roohandeh, R. J. Mol. Liq. 2012, 167, 69.
Zare, A.; Yousofi, T.; Moosavi-Zare, A. R. RSC Adv. 2012, 2, 7988.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary information file containing Table S1, Table S2, 1H, 13C NMR, and mass spectra of the new compounds is available at the journal website at http://link.springer.com/journal/10593.
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2018, 54(11), 1056–1060
Electronic supplementary material
ESM 1
(PDF 5203 kb)
Rights and permissions
About this article
Cite this article
Pouramiri, B., Far, R.G. & Zahedifar, M. Acidic ionic liquids: highly efficient catalysts for one-pot four-component synthesis of pyrazolo[1,2-b]phthalazines under solvent-free conditions. Chem Heterocycl Comp 54, 1056–1060 (2018). https://doi.org/10.1007/s10593-018-2391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-018-2391-y