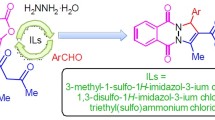
Abstract
Two novel room-temperature disulfonic acid functionalized ionic liquids derived from 3,5-dimethyl-1H-pyrazole consisting of chloride and trichlorostannate anions, 3,5-dimethyl-1,2-disulfonic acid-1H-pyrazolium chloride and 3,5-dimethyl-1,2-disulfonic acid-1H-pyrazolium trichlorostannate, have been synthesized, characterized and evaluated for their catalytic efficiency in the synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones by the one-pot, three-components reaction of phthalhydrazide, an aromatic aldehyde and malononitrile or ethyl cyanoacetate under solvent-free conditions. The results have demonstrated high catalytic activity of these novel ionic liquids containing acidic SO3H groups in producing high yields of the desired products in short reaction time. The ionic liquids can be used at least four times without any noticeable decrease in catalytic activity.


Similar content being viewed by others
REFERENCES
Ugi, I., Pure Appl. Chem., 2001, vol. 73, no. 1, p. 187. https://doi.org/10.1351/pac200173010187
Mirzaie, Y., Lari, J., Vahedi, H., Hakimi, M., Nakhaei, A., and Rezaeifard, A., J. Mex. Chem. Soc., 2017, vol. 61, no. 1, p. 35.
Kazemi, E., Davoodnia, A., Nakhaei, A., Basafa, S., and Tavakoli-Hoseini, N., Adv. J. Chem. A, 2018, vol. 1, no. 2, p. 96. https://doi.org/10.29088/SAMI/AJCA.2018.1.96104
Nakhaei, A., Davoodnia, A., and Nakhaei, H., J. Chem. Rev., 2019, vol. 1, no. 2, p. 139. https://doi.org/10.33945/SAMI/JCR.2019.2.5
Toure, B.B. and Hall, D.G., Chem. Rev., 2009, vol. 109, no. 9, p. 4439. https://doi.org/10.1021/cr800296p
Al’-Assar, F., Zelenin, K., Lesiovskaya, E., Bezhan, I., and Chakchir, B., Pharm. Chem. J., 2002, vol. 36, no. 11, p. 598. https://doi.org/10.1023/A:1022665331722
Nakhaei, A., Davoodnia, A., and Morsali, A., Russ. J. Phys. Chem. A, 2018, vol. 92, no. 2, p. 271. https://doi.org/10.1134/S0036024418020036
Reddy, M.V. and Jeong, Y.T., Tetrahedron Lett., 2013, vol. 54, no. 27, p. 3546. https://doi.org/10.1016/j.tetlet.2013.04.109
Karthikeyan, G. and Pandurangan, A., J. Mol. Catal. A: Chem., 2012, vols. 361–362, p. 58. https://doi.org/10.1016/j.molcata.2012.05.003
Azarifar, A., Nejat-Yami, R., and Azarifar, D., J. Iran. Chem. Soc., 2013, vol. 10, no. 2, p. 297. https://doi.org/10.1007/s13738-012-0159-3
Ghahremanzadeh, R., Shakibaei, G.I., and Bazgir, A., Synlett, 2008, vol. 2008, no. 08, p. 1129. https://doi.org/10.1055/s-2008-1072716
Vafaee, A., Davoodnia, A., Pordel, M., and Bozorgmehr, M.R., Orient. J. Chem. , 2015, vol. 31, no. 4, p. 2153. https://doi.org/10.13005/ojc/310437
Kidwai, M., and Chauhan, R., J. Heterocycl. Chem., 2014, vol. 51, no. 6, p. 1689. https://doi.org/10.1002/jhet.1809
Maleki, B., Barat Nam Chalaki, S., Sedigh Ashrafi, S., Rezaee Seresht, E., Moeinpour, F., Khojastehnezhad, A., and Tayebee, R., Appl. Organomet. Chem., 2015, vol. 29, no. 5, p. 290. https://doi.org/10.1002/aoc.3288
Raghuvanshi, D.S. and Singh, K.N., Tetrahedron Lett., 2011, vol. 52, no. 43, p. 5702. https://doi.org/10.1016/j.tetlet.2011.08.111
Nabid, M.R., Rezaei, S.J.T., Ghahremanzadeh, R., and Bazgir, A., Ultrason. Sonochem., 2010, vol. 17, no. 1, p. 159. https://doi.org/10.1016/j.ultsonch.2009.06.012
Safaei-Ghomi, J., Shahbazi-Alavi, H., Ziarati, A., Teymuri, R., and Saberi, M.R., Chin. Chem. Lett., 2014, vol. 25, no. 3, p. 401. https://doi.org/10.1016/j.cclet.2013.11.046
Shaterian, H.R. and Mohammadnia, M., J. Mol. Liq., 2012, vol. 173, p. 55. https://doi.org/10.1016/j.molliq.2012.06.007
Davoodnia, A., Yadegarian, S., Nakhaei, A., and Tavakoli-Hoseini, N., Russ. J. Gen. Chem., 2016, vol. 86, no. 12, p. 2849. https://doi.org/10.1134/S1070363216120495
Rad-Moghadam, K., Taghizadeh Valadi, A., and Alipour, A., Appl. Organomet. Chem., 2014, vol. 28, no. 3, p. 146. https://doi.org/10.1002/aoc.3099
Greaves, T.L. and Drummond, C.J., Chem. Rev., 2008, vol. 108, no. 1, p. 206. https://doi.org/10.1021/cr068040u
Nakhaei, A., Davoodnia, A., and Yadegarian, S., Iran. J. Catal., 2018, vol. 8, no. 1, p. 47.
Nakhaei, A., Davoodnia, A., and Yadegarian, S., Iran. J. Chem. Chem. Eng., 2018, vol. 37, no. 3, p. 33. https://doi.org/10.30492/IJCCE.2018.27405
Nakhaei, A., Ramezani, S., Shams-Najafi, S.J., and Farsinejad, S., Lett. Org. Chem., 2018, vol. 15, no. 9, p. 739. https://doi.org/10.2174/1570178615666171226162735
Nakhaei, A., Curr. Catal., 2018, vol. 7, no. 1, p. 72. https://doi.org/10.2174/2211544706666171010155918
Nakhaei, A., and Davoodnia, A., Chin. J. Catal., 2014, vol. 35, no. 10, p. 1761. https://doi.org/10.1016/S1872-2067(14)60174-1
Nakhaei, A., Davoodnia, A., and Morsali, A., Res. Chem. Intermediat., 2015, vol. 41, no. 10, p. 7815. https://doi.org/10.1007/s11164-014-1861-9
Nakhaei, A., Davoodnia, A., and Yadegarian, S., Iran. Chem. Commun., 2018, vol. 6, no. 4, p. 334. https://doi.org/10.30473/ICC.2018.3892
Nakhaei, A., Shojaee, S., Yaghoobi, E., and Ramezani, S., Heterocycl. Lett., 2017, vol. 7, no. 2, p. 323.
Rohaniyan, M., Davoodnia, A., and Nakhaei, A., Appl. Organomet. Chem., 2016, vol. 30, no. 8, p. 626. https://doi.org/10.1002/aoc.3479
Davoodnia, A. and Nakhaei, A., Synth. React. Inorg. M., 2016, vol. 46, no. 7, p. 1073. https://doi.org/10.1080/15533174.2015.1004419
Lee, B., Kang, P., Lee, K.H., Cho, J., Nam, W., Lee, W.K., and Hur, N.H., Tetrahedron Lett., 2013, vol. 54, no. 11, p. 1384. https://doi.org/10.1016/j.tetlet.2012.12.106
Pradhan, K., Paul, S., and Das, A.R., Monatsh Chem., 2014, vol. 145, no. 8, p. 1343. https://doi.org/10.1007/s00706-014-1195-8
ACKNOWLEDGMENTS
The authors express their gratitude to the Islamic Azad University, Mashhad Branch for its financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Vafaee, A., Davoodnia, A., Nakhaei, A. et al. Two Novel Pyrazole Derived Ionic Liquids Based on Chloride and Trichlorostannate Anions: Preparation, Characterization, and Evaluation of Their Catalytic Activity in the Synthesis of 1H-Pyrazolo[1,2-b]phthalazine-5,10-diones. Russ J Gen Chem 91, 273–278 (2021). https://doi.org/10.1134/S1070363221020158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221020158