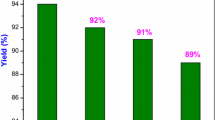
Abstract
We describe regioselective synthesis of pyrazolo[3,4-b]quinoline derivatives by multicomponent reaction of dimedone, 5-aminopyrazolone, and aromatic aldehydes in presence of H3PW12O40 as catalyst. When this multicomponent reaction was investigated without catalyst under reflux conditions, a mixture of products was obtained, while the reaction successfully proceeded to formation of pyrazolo[3,4-b]quinoline in presence of H3PW12O40. Good product yield, short experimental time, and low-cost catalyst provide convenient synthesis for formation of pyrazolo[3,4-b]quinoline pharmacological compounds.


Similar content being viewed by others
References
T. Ueda, H. Mase, N. Oda, I. Ito, Chem. Pharm. Bull. 29, 3522 (1981)
C. Malvar Ddo, R.T. Ferreira, R.A. de Castro, L.L. de Castro, A.C. Freitas, E.A. Costa, I.F. Florentino, J.C. Mafra, G.E. de Souza, F.A. Vanderlinde, Life Sci. 95, 81 (2014)
M. Sanchez-Moreno, C. Marin, P. Navarro, L. Lamarque, E. Garcia-Espana, C. Miranda, O. Huertas, F. Olmo, F. Gomez-Contreras, J. Pitarch, F. Arrebola, J. Med. Chem. 55, 4231 (2012)
X. Fan, X. Zhang, L. Zhou, K.A. Keith, E.R. Kern, P.F. Torrence, Bioorg. Med. Chem. Lett. 16, 3224 (2006)
S.-L. Wang, Y.P. Liu, B.H. Xu, X.H. Wang, B. Jiang, S.J. Tu, Tetrahedron 67, 9417 (2011)
W. Anna, B. Lilianna, Mini Rev. Org. Chem. 12, 298 (2015)
S.T. Selvi, V. Nadaraj, S. Mohan, R. Sasi, M. Hema, Bioorg. Med. Chem. 14, 3896 (2006)
A. Kamal, J.R. Tamboli, V.L. Nayak, S.F. Adil, M.V. Vishnuvardhan, S. Ramakrishna, Bioorg. Med. Chem. Lett. 23, 3208 (2013)
S. Taliani, I. Pugliesi, E. Barresi, S. Salerno, C. Marchand, K. Agama, F. Simorini, C. La Motta, A.M. Marini, F.S. Di Leva, L. Marinelli, S. Cosconati, E. Novellino, Y. Pommier, R. Di Santo, F. Da Settimo, J. Med. Chem. 56, 7458 (2013)
R.L. Siegel, K.D. Miller, A. Jemal, CA Cancer J. Clin. 66, 7 (2016)
S. Rádl, V. Zikán, F. Šmejkal, Collect. Czech. Chem. Commun. 50, 1057 (1985)
P. Siminoff, R.R. Crenshaw, Antimicrob. Agents Chemother. 11, 571 (1977)
R.G. Stein, J.H. Biel, T. Singh, J. Med. Chem. 13, 153 (1970)
P.K. Sharma, K. Singh, S. Kumar, P. Kumar, S.N. Dhawan, S. Lal, H. Ulbrich, G. Dannhardt, Med. Chem. Res. 20, 239 (2011)
C. Kalinski, H. Lemoine, J. Schmidt, C. Burdack, J. Kolb, M. Umkehrer, G. Ross, Synthesis 2008, 4007 (2008)
I. Ugi, A. Dömling, W. Hörl, Endeavour 18, 115 (1994)
J.E. Biggs-Houck, A. Younai, J.T. Shaw, Curr. Opin. Chem. Biol. 14, 371 (2010)
T. Zarganes-Tzitzikas, A.L. Chandgude, A. Domling, Chem. Rec. 15, 981 (2015)
M. Manpadi, P.Y. Uglinskii, S.K. Rastogi, K.M. Cotter, Y.S. Wong, L.A. Anderson, A.J. Ortega, S. Van Slambrouck, W.F. Steelant, S. Rogelj, P. Tongwa, M.Y. Antipin, I.V. Magedov, A. Kornienko, Org. Biomol. Chem. 5, 3865 (2007)
P. Acosta, E. Butassi, B. Insuasty, A. Ortiz, R. Abonia, S.A. Zacchino, J. Quiroga, Molecules 20, 8499 (2015)
V.A. Chebanov, V.E. Saraev, S.M. Desenko, V.N. Chernenko, I.V. Knyazeva, U. Groth, T.N. Glasnov, C.O. Kappe, J. Org. Chem. 73, 5110 (2008)
M. Zahedifar, H. Sheibani, Chem. Heterocycl. Compd. 52, 41 (2016)
M. Zahedifar, H. Sheibani, Aust. J. Chem. 67, 1201 (2014)
H. Sheibani, V. Saheb, M. Rezaei, M. Zahedifar, Lett. Org. Chem. 11, 126 (2014)
M. Zahedifar, H. Sheibani, Res. Chem. Intermed. 41, 105 (2013)
M. Zahedifar, R. Razavi, H. Sheibani, J. Mol. Struct. 1125, 730 (2016)
F. Alinaghizadeh, M. Zahedifar, M. Seifi, H. Sheibani, J. Braz. Chem. Soc. 27, 663 (2016)
M. Abaszadeh, H. Sheibani, K. Saidi, Aust. J. Chem. 63, 92 (2010)
I. Drizin, R.J. Altenbach, S.A. Buckner, K.L. Whiteaker, V.E. Scott, J.F. Darbyshire, V. Jayanti, R.F. Henry, M.J. Coghlan, M. Gopalakrishnan, W.A. Carroll, Bioorg. Med. Chem. 12, 1895 (2004)
Acknowledgements
The authors express appreciation to the University of Jiroft Faculty Research Committee and Shahid Bahonar University of Kerman for supporting this investigation. This research was supported by the University of Jiroft under grant no. 95-3813-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zahedifar, M., Shojaei, R. & Sheibani, H. Convenient regioselective reaction in presence of H3PW12O40: synthesis and characterization of pyrazolo[3,4-b]quinoline-3,5-diones. Res Chem Intermed 44, 873–882 (2018). https://doi.org/10.1007/s11164-017-3141-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3141-y