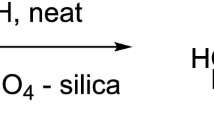
An improved method for the preparation of 6-sulfamoylsaccharin (3-oxo-2,3-dihydro-1,2-benzo-thiazole-6-sulfonamide 1,1-dioxide) has been developed and studies of its possible direct alkylation have been carried out. It was shown that alkylation occurs regioselectively at the isothiazoline ring nitrogen atom.
Similar content being viewed by others
References
R. Okachi, H. Niino, K. Kitaura, K. Mineura, Y. Nakamizo, Y. Murayama, T. Ono, and A. Nakamizo, J. Med. Chem., 28, 1772 (1985).
D. Goukassian, S. M. Sanz-González, I. Pérez-Roger, J. F. de Mora, J. Ureña, and V. Andrés, Br. J. Pharmacol., 132, 1597 (2001).
A. L. Vaccarino, D. Paul, P. K. Mukherjee, E. B. Rodríguez de Turco, V. L. Marcheselli, L. Xu, M. L. Trudell, J. M. Minguez, M. P. Matía, C. Sunkel, J. Alvarez-Builla, and N. G. Bazan, Bioorg. Med. Chem., 15, 2206 (2007).
M. Rami, J.-Y. Winum, A. Innocenti, J.-L. Montero, A. Scozzafava, and C. T. Supuran, Bioorg. Med. Chem. Lett., 18, 836 (2008).
C. T. Supuran, A. Scozzafava, and J. Conway, Carbonic Anhydrase: Its Inhibitors and Activators, CRC Press, Boca Raton, New York, London (2004).
A. A. Spryskov and T. I. Yakovleva, Zh. Obshch. Khim., 25, 783 (1955).
E. Yuriev, D. C. M. Kong, and M. N. Iskander, Eur. J. Med. Chem., 39, 835 (2004).
A. V. Tarasov, P. O. Yablonskii, and Yu. A. Moskvichev, Khim. Geterotsikl. Soedin., 126 (2003). [Chem. Heterocycl. Comp., 39, 119 (2003)].
H. Kamogawa, S. Yamamoto, and M. Nanasawa, Bull. Chem. Soc. Jpn., 55, 3824 (1982).
L. Xu, H. Shu, Y. Liu, S. Zhang, and M. L. Trudell, Tetrahedron, 62, 7902 (2006).
V. N. Voshchula, S. V. Tolkunov, M. Yu. Zubritskii, and V. I. Dulenko, Khim. Geterotsikl. Soedin., 1414 (1988). [Chem. Heterocycl. Comp., 24, 1175 (1988)].
Z. Jakopin and M. S. Dolenc, Synth. Commun., 40, 2464 (2010).
G. H. Hamor, J. Pharm. Sci., 51, 1109 (1962).
This work was carried out with the financial support of the European Social Fund (No. 2009/0203/1DP/1.1.1.2.0/APIA/VIAA/023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1865-1868, December, 2011.
Rights and permissions
About this article
Cite this article
Ivanova, E.M., Simin, E.Y., Vozny, I.V. et al. Synthesis of 6-sulfamoylsaccharin and study of its reactivity in alkylation reactions. Chem Heterocycl Comp 47, 1561–1564 (2012). https://doi.org/10.1007/s10593-012-0948-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0948-8