Abstract
Fischer glycosylation is typically the chemical reaction of a monosaccharide and an alcohol in presence of an acidic catalyst to afford glycosides in pyranosidic and furanosidic forms. This reaction is still applied today for the synthesis of specialized glycosides, and optimization and modification of the method have continued since its discovery by Emil Fischer in the 1890s. This review presents advancements in Fischer glycosylation described in literature of the past 15 years and its implementation in modern chemical methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carbohydrates play a major role in the development and functionality of organisms. For many years, research exploring carbohydrates was regarded as less interesting than most other topics in biochemistry. However, with time, this view has changed and the vital role that carbohydrates play in many processes is attracting more research interest [1]. Due to the heightened interest in glycoscience, the importance of glycosides in many biological processes has also come to light. This shows a clear need for strategies to synthesize specific glycosides. Significant effort has been invested in research into various synthetic strategies such as the Koenigs–Knorr reaction, the more versatile trichloracetimidate method, as well as others [2,3,4,5,6,7,8]. Despite the number of different protocols reported, the approach first mentioned and developed in the 1890s by the German chemist Emil Fischer remains a very valuable method for synthesis of simple glycosides. In the classical approach described by Fischer, the sugar is dissolved in an alcohol in the presence of a strong acid. It is known that, owing to the presence of water as a byproduct, the formed oxocarbenium ion can react back to the reducing sugar. This unwanted effect can be addressed by running the reaction in an excess of alcohol to shift the equilibrium towards the desired end product. The reaction delivers a mixture of furanosides and pyranosides, with furanosides being the product of kinetic control while the more stable pyranosides can be derived through thermodynamic control. The basic mechanism of Fischer glycosylation is described throughout the scientific and educational literature [9,10].
While the main benefit of Fischer glycosylation lies in its simplicity, the approach suffers from limitations such as the need for strong Lewis acids and long reaction times, as well as the production of a mixture of products of furanosides and pyranosides as well as their anomeric forms [11,12]. These shortcomings show the need for new approaches towards Fischer glycosylation to overcome these limitations. In this review, we look at different approaches such as the use of new catalysts and technologies such as microwave or flow chemistry.
2 Use of Different Catalysts and Additives for Fischer Glycosylation
Several reagents have been successfully implemented as new catalysts for Fischer glycosylation. While increasing product yield and selectivity was generally a desired effect, catalysts have also been chosen on the basis of their ecofriendliness and ability to synthesize sugars, without the need for protecting groups. Modifications of the classic Fischer glycosylation method have also been applied, for example, adding organic additives to the reaction to increase yield or creating a micellar reaction system to increase the solubility of carbohydrates in alcohols.
2.1 Sulfuric Acid Immobilized on Silica for Synthesis of Glycosides from Free Sugars
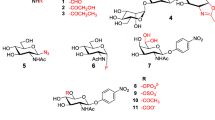
Sulfuric acid immobilized on silica (H2SO4-silica) is a catalyst that was used by Roy et al. to shorten the reaction time and reduce the amount of alcohol required to prepare glycosides from free sugars by Fischer glycosylation (Fig. 1) [13].
In a first approach, the authors added d-glucose and H2SO4-silica to propargyl alcohol at 65 °C and observed the reaction over time. After cooling to room temperature, excess propargyl alcohol was eluted with dichloromethane (CH2Cl2) over a silica gel column, followed by elution of the product with a dichloromethane–methanol (CH2Cl2–MeOH) (15:1) mixture, providing the desired propargyl glycoside. The product was per-O-acetylated with Ac2O and H2SO4-silica for 1H and 13C nuclear magnetic resonance (NMR) analysis (Table 1) [13].
No formation of furanosides or acyclic acetals as byproducts has been observed. Method C provided good anomeric selectivity and good yields in a short time compared with methods A and B. Using method C, several propargyl glycosides were prepared with different reducing sugars (Table 2) [13].
The reaction yielded glycosides between 69% and 83% with α-isomer-favored selectivity. Using the same method, the reaction was performed with d-glucose and various alcohols (Table 3).
The reaction yielded glycosides between 75% and 82%, and again the α-isomer was the favored anomer. The strategy provided by the authors is applicable for large-scale preparations, and the purification of the product only requires filtration [13].
2.2 Acid Zeolites for Glycosylation of N-Acetylgalactosamine
Rauter et al. used HY, HZSM-5, and HBEA acid zeolites as catalysts for glycosylation of N-acetylgalactosamine (GalNAc) to β-galactofuranosides and β-galactopyranosides. Acidic zeolites are solid, ecofriendly catalytic materials that can differentiate molecules based on their size and form because of their pore and zeolite channels [14]. Within the pores, high selectivity and product concentrations can be achieved. For glycosylation of GalNAc with methanol, four acid zeolites with different characteristics were examined (Fig. 2, Table 4) [15].
For the reaction, activated zeolite was added to GalNAc in dry methanol and the mixture was refluxed under stirring for 48 h at 60 °C. After filtering the zeolite and evaporating the solvent, the residue was dissolved in pyridine and acetic anhydride was added. The mixture was stirred for 24 h at room temperature. After work-up, compounds 15 and 18 could be isolated in different ratios (Table 5) [15].
Derivative 15 was found to be favored for all used catalysts. The regioselectivity for the furanoside/pyranoside form was 2:1 for the large-pore zeolites (HY and HBEA) and 14:1 for the medium-pore zeolite (HZSM-5). HY (3.1) yielded the highest amount of product, with only 2% of starting material being recovered. The group suggested that the high concentration of Brønsted acid sites and the higher aluminum content, which leads to higher hydrophilicity, are the reasons for the high efficiency of this catalyst. Using HY (3.1) as catalyst, methyl glycosylation of GalNAc, followed by in situ acetonation, was achieved, which resulted in 16 as the major product in 43% yield. This confirmed that the zeolite increases the selectivity for synthesis of a furanoside form [15].
2.3 Ionic-Liquid-Promoted Fischer Glycosylation
In 2009, Jaques Auge et al. reported the use of ionic liquids together with catalytic amounts of various triflate-salt-based Lewis acids as a system for Fischer glycosylation of several monosaccharides with a variety of shorter and longer alcohols [16]. In their work, they could show that, when using ionic liquids based on 1-butyl-3-methylimidazolium, 1,3-dimethylimidazolium, and 1,2,3-trimethylimidazolium cations with a variety of different anions, the performance of the Fischer glycosylation could be increased significantly compared with reactions in neat alcohols. Additionally, the amount of alcohol could be reduced down to 1 equiv. and the amount of Lewis acid could be reduced down to 1 mol.%, while the yields could be doubled in some cases compared with classical Fischer glycosylation in neat alcohols (high excess) using 5 mol.% Lewis acids.
Another advantage mentioned by those authors was the reusability of the ionic liquids without influencing the reaction performance (at least three cycles), making this process more ecofriendly than other state-of-the-art Fischer glycosylation reactions.
2.4 Sulfamic Acid for Synthesis of Glycosides from Unprotected Sugars
In search of an ecofriendly catalyst for Fischer glycosylation of unprotected sugars, Guchhait et al. successfully used sulfamic acid (H3NSO3) to prepare alkyl glycosides. Sulfamic acid is inexpensive, nonvolatile, noncorrosive, and moderately acidic (pKa = 1.0), which prevents the decomposition of the product. The mentioned research group confirmed the catalytic activity of sulfamic acid by the reaction of d-glucose with different quantities of benzyl alcohol in presence of sulfamic acid at 80 °C for 5 h under neat conditions, which led to the formation of an anomeric mixture (α:β = 6:1) of 20 in 81% yield (Fig. 3) [17].
After removal of the solvent, the pure compound was obtained by passing the crude product through a pad of silica. In preparation for 1H and 13C NMR analysis, the compound was acetylated. After these first results, five unprotected monosaccharides (d-glucose, d-mannose, d-galactose, N-acetyl-d-glucosamine, and l-rhamnose) were treated with several aliphatic alcohols under similar reaction conditions. The resulting alkyl glycosides were obtained in satisfactory yield (70–85%), and the major isomer in all cases was the α-isomer. The reaction of sugars with primary and secondary alcohols resulted in alkyl glycosides as products, but with tertiary alcohols no product was formed, which may be because of the polymerization of tertiary alcohols under acidic conditions. A selection of results of these experiments are presented in Table 6 [17].
Additionally, different catalysts for the reaction with d-glucose and benzyl alcohol were compared (Table 7).
Triflic acid (CF3SO3H) yielded a similar amount of product as sulfamic acid in the same amount of time but is corrosive and moisture sensitive, thus sulfamic acid has been shown to be the favorable catalyst for these kinds of reactions.
2.5 Synthesis of α-Linked Mannobioses with Hydrochloric-Acid-Assisted Fischer-Type Glycosylation
Ajisaka et al. developed an acid-assisted reverse hydrolysis reaction based on Fischer glycosylation for synthesis of α-linked mannobioses to replace the more expensive and less efficient enzyme-assisted reverse hydrolysis reaction (Fig. 4) [18].
The alcoholic solution, which is typical for Fischer glycosylation, was replaced with aqueous solution with a very high concentration (83% w/w) of d-mannose to shift the equilibrium towards hydrolysis. To a solution containing 5 g d-mannose and 1 mL water, 240 μL 12.5 M HCl was added to achieve a final concentration of 0.5 M HCl. The mixture was stirred for 65 h at 60 °C. Then, the solution was diluted with water and neutralized with 0.1 M NaOH. To hydrolyze the β-linkages, β-mannosidase was added, and the solution was incubated at 37 °C for 24 h. The mannobioses were separated by activated carbon column chromatography, and their structures were identified by 13C NMR spectroscopy. Amounts and yields of the separated products are summarized in Table 8 [18].
Three different kinds of mannobioses (table entries 2–4) were obtained in moderate yield (45% in total) in one reaction. Compound 33 was a by-product of the reaction. The reaction is based on an equilibrium between mono-, di-, and higher oligosaccharides and can be carried out repeatedly to create an efficient reaction system for the preparation of α-linked mannobioses [18].
2.6 Furandicarboxylic Acid and Its Ester as Organic Additive for Synthesis of Alkyl Polyglucosides
Van Es et al. also used sulfuric acid as catalyst for Fischer glycosylation of d-glucose in decanol to prepare alkyl polyglucosides (APG), specifically decyl glucosides, for cosmetic use and as surfactants. They used furandicarboxylic acid (FDCA) and its mono-n-decyl-ester (C10-FDCA) as environmentally friendly, organic additives to increase product yield and limit side reactions to reduce the coloration of the reaction, therefore reducing the requirement for exhaustive purification [19].
For the glycosylation, d-glucose was added to a solution containing decanol and sulfuric acid. The mixture was heated at 95 °C and 50 mbar for 2 h. The crude product was cooled to 80 °C, and sodium carbonate (Na2CO3) was added at atmospheric pressure. The resulting medium was analyzed by gas chromatography (GC). The reaction was carried out without additives as reference, and the effect of several additives on the reaction was compared (Table 9) [19].
Adding FDCA at 2.5 mol.% had the highest positive impact on the yield, increasing it to 59% and lowering the optical density (in this case a degree for the degradation of glucose during the reaction by measuring the coloration) to 0.057 compared with the reference reaction. C10-FDCA at 2.5 mol.% had the highest positive impact on optical density, lowering it to 0.016 at 52% yield. Citric and isophthalic acid only yielded 38% product, and while citric acid lowered the optical density to 0.037, isophthalic acid increased it to 1.4, making these additives less favorable than those with FDCA and C10-FDCA [19].
The polyglucoside mixtures obtained by the reactions with FDCA and C10-FDCA as additives at 2.5 mol.% and 5 mol.% were compared with an n-octyl/n-decyl polyglucoside product that is often used in detergent industry, based on several physiochemical properties that are indicators for the performance of surfactants (Table 10) [19].
The surface tension at critical micelle concentration (CMC) was the same for all glycoside mixtures (28 mN m−1) except for the octyl/decyl polyglucosides (26 mN m−1). The CMC of the glucosides obtained by adding FDCA were slightly lower (424 mg/L and 483 mg/L) than the CMC of the glucosides obtained without additives (500 mg/L) and much lower compared with the CMC of the octyl/decyl polyglucosides (963 mg/L). The CMC of glucosides obtained by adding C10-FDCA were slightly higher (614 mg/L and 596 mg/L) than the CMC of glucosides obtained without additives. A lower CMC means that a lower concentration of glycosides is needed for an efficient detergent, which is desirable for a more economic process. The foam volume varies slightly between the glucoside mixtures (430–460 mL), but using FDCA as additive increases the foam stability to 80%, while using C10-FDCA decreases it to 60%. Low foam volume and stability are favorable for detergents but unfavorable for cosmetic application. The wetting times of the glycoside mixtures prepared by the authors were all much lower (26–47 s) than in the case of the industrial octyl/decyl polyglucosides (196 s) [19].
2.7 Ammonium Chloride for Synthesis of Alkyl Glycosides
Sharma et al. used ammonium chloride (NH4Cl) as catalyst to create glycosides of unprotected monosaccharides and sugar acids under solvent-free conditions with different alcohols. Additionally, the group analyzed the immunomodulatory activity of the products, to find out whether they could potentially be used as adjuvants for vaccines. For the reaction, NH4Cl was added to a solution of d-glucose and decanol, and the mixture was heated at 90 °C for 6 h. After completion of the reaction, the mixture was filtered through a silica pad with 0–15% methanol (MeOH) in dichloromethane (CH2Cl2) to obtain 34 as product [20].
The structure and anomeric ratio of the products were identified by 1H and 13C NMR spectroscopy. The reaction showed excellent yield (72%), with 34α as the preferred derivative (α:β = 2.7:1). The reaction was quicker and more efficient compared with other reaction conditions (Table 11) [20].
NH4Cl was then used to catalyze the glycosylation of different reducing sugars (donors) with decanol and additionally for the glycosylation of d-glucose with several alcohols (acceptors) with different chain lengths, under the reaction conditions described above (Table 12).
The glucosides in Table 12 had good to excellent yield (65–72%) with high anomeric selectivity, and the glycosylation of d-mannose resulted only in α-isomers.
d-Glucuronic acid and N-acetylneuraminic acid were analyzed under the same conditions to see whether their corresponding glycosides were formed (Table 13).
d-Glucuronic acid reacted well with decanol and propargyl alcohol, and the corresponding glycosides 41 and 42 were formed in good yield and anomeric selectivity. The reaction of N-acetylneuraminic acid yielded only an esterified product, instead of its corresponding glycoside, which has not been fully characterized.
2.8 Bismuth Nitrate Pentahydrate for Synthesis of Glycosides from Unprotected and Unactivated Sugars
Polanki et al. proposed bismuth nitrate pentahydrate (Bi(NO3)3·5H2O) as another ecofriendly catalyst for Fischer glycosylation of unprotected and inactivated sugars. Bismuth nitrate is very accessible and inexpensive and has low toxicity [21]. In preliminary experiments, bismuth nitrate was added to a solution of d-glucose and propargyl alcohol and the mixture was stirred at 60 °C under nitrogen until completion as determined by thin-layer chromatography (TLC). Excess alcohol was removed under vacuum, and the crude product was eluted with a dichloromethane–methanol mixture in a short bed of celite to provide the desired glycoside as an anomeric mixture (α:β = 10:1) in 83% yield. 1H NMR spectroscopy was carried out with acetylated crude product to obtain the ratio of α- and β-anomers (Fig. 5) [22].
The reaction (Fig. 6) was carried out with several other sugars and alcohols under the same conditions to prepare a series of different glycosides. Table 14 shows selected results of the reaction series.
The products were obtained in good to excellent yield and anomeric selectivity, with the α-anomer being predominant in all cases. The reaction method was compared with other previously reported methods for Fischer glycosylation based on temperature, reaction time, yield, and anomeric ratio (Table 15) [13,17,22,23].
The glycosylation reaction catalyzed with bismuth nitrate yielded products with better anomeric selectivity than previously reported methods.
The research group also developed an automated flash liquid chromatography method to isolate the α- and β-anomers of glycosides using silver-nitrate-impregnated silica gel as an alternative to the classical silica gel column chromatography, which often does not perform well when trying to separate α- and β-anomers of glycosides. The peaks were detected with a UV detector and evaporative light scattering detector (ELSD) and eluted with a mixture of petroleum ether and ethyl acetate in gradient mode. All the prepared glycosides were separated to obtain pure α- and β-anomers, except the propargyl glycosides that formed a complex mixture with silver nitrate, which could not be separated. These complex mixtures were separated by flash chromatography under the same conditions [22].
2.9 Boronic Acids as Phase-Transfer Reagents for Fischer Glycosylation Catalyzed by Camphorsulfonic Acid
Manhas et al. used boronic acids as phase-transfer reagents for Fischer glycosylation of sugars catalyzed by camphorsulfonic acid (CSA) in organic low-polarity solvents to increase the rate of the reaction, prepare functionalized glycosides, and conduct selective transformations in carbohydrate mixtures. First, the effects of boronic acid were determined with d-mannose in dichloroethane and heptane as solvents (Table 16). For the reaction, d-mannose, CSA, boronic acid, octanol and dichloroethane (DCE) or heptane, respectively, were added in a vial and purged with argon. The mixture was stirred at 80 °C for 24 h. Afterwards, the solvent was evaporated, and an aqueous solution of sodium carbonate (Na2CO3) and sorbitol was added to the mixture to split the boronic ester groups and separate the glycoside product from the boronic acid. The product was acetylated, and the anomeric ratio was determined by 1H NMR spectroscopy [24].
The reactions without boronic acids yielded low amounts of product. The main product of the reactions in presence of boronic acid was 48α, which is unusual, since pyranosides are the products of thermodynamic control and predominant in classic Fischer glycosylation. Synthesizing furanosides with Fischer glycosylation is usually not trivial as the level of selectivity depends on the reaction conditions as well as the chosen substrate and catalyst [25], but there have been previously reported methods that used boronic esters or borate intermediates to prepare furanosides [26,27]. The highest increase in yield (70% of 48α) was achieved using 2 equiv. phenylboronic acid relative to d-mannose in heptane. Letting methyl α-mannopyranoside react under the conditions of Table 16, entry 4 resulted in the formation of the same α-mannofuranoside at 65% yield, but at a slower rate than when using d-mannose as substrate. Several other sugars were subjected to the same reaction conditions described above, using DCE as solvent and adding phenylboronic acid [24].
Generally, the formation of boronic esters at nonanomeric OH groups was favored, except when d-glucose was used as substrate, where a furanoside-derived boronic ester was formed involving the anomeric OH group and therefore suppressing the glycosylation reaction. Utilizing N-acetyl-d-glucosamine instead of d-glucose prevents the participation of the anomeric OH in the formation of the boronic ester, and glycosylation could occur, leading to a mixture of furanosides and pyranosides. d-Mannose, l-rhamnose, d-lyxose, and d-ribose formed furanosides, supposedly due to the stability of boronic ester derived from cis-1,2-diol moieties on five-membered rings. Substrates without those moieties formed pyranosides with boronic esters involving the 4,6- or 3,4-diol groups. Utilizing the boronic ester groups of the boronic ester intermediates as protective groups, several benzoylated glycoside derivatives were also prepared. Therefore, benzoyl chloride and pyridine were added to the boronic ester intermediates before phase-switching deprotection [24].
Finally, d-mannose was mixed with d-glucose and/or d-galactose before carrying out a reaction under the conditions described above. In all cases, 48α was formed selectively in good yield without glucose- or galactose-derived products. The authors chose the ratio of the three-component mixture to mimic the sugar content of sprucewood-derived hemicelluloses, which are a renewable chemical resource [28]. To synthesize products from carbohydrate mixtures, selective reactions, similar to the reaction presented by the mentioned research group, may be beneficial.
2.10 Micellar Effect on Synthesis of Alkyl Glucosides by Fischer Synthesis
Nowicki et al. created a novel approach for Fischer glycosylation of unprotected d-glucose based on a micellar reaction system by using dodecylbenzenesulfonic acid (DBSA), which acts as both surfactant and catalyst for the reaction. A biphasic reaction system, e.g., a microemulsion, increases the reaction surface through the creation of micelles, which circumvents the poor solubility of alkyl glucosides in aliphatic alcohols [29]. The authors took advantage of the separation of reactants through micelles and the entrapment of water inside the micelles [30,31]. Figure 7 shows the proposed reaction pathway [32].
Synthesis of alkyl glucosides via the “microemulsion” route [32]
Several experiments were carried out with different aliphatic alcohols, at different temperatures, and in the presence of additional water to confirm the catalytic potential of DBSA. For the reaction, d-glucose was added to the corresponding alcohol and DBSA. The mixture was agitated at 700 rpm for 24 h at the desired temperature. The products were analyzed with high-performance liquid chromatography (HPLC), gas chromatography–mass spectrometry (GC–MS), and gel permeation chromatography (GPC). To assess the impact of APGs on glucose conversion, the commercial APG Glucopon and methanesulfonic acid (CH3SO3H) were used instead of DBSA in one experiment (Table 17) [32].
Conversion of d-glucose in octanol decreased with increasing temperature when additional water was present in the reaction. The opposite effect was observed when no additional water was added, reaching 99% conversion at 80 °C. Similar d-glucose conversions are obtained with decanol (98.2%) and dodecanol (97.5%). Using an APG as surfactant and a homogeneous sulfonic acid as catalyst resulted in much lower conversion of glucose (45.7%). The authors propose that this is due to water and APGs being fully miscible and therefore not forming micelles to capture water molecules. The effect of different amounts of DBSA on sugar conversion has been tested (Table 18) [32].
Lower loads of catalyst resulted in lower glucose conversion. The highest conversion (99%) was achieved with 5 mol.% DBSA.
The water molecules resulting from the glycosylation reaction were captured in the hydrophilic interior of the cluster in dispersed phase, while the alkyl glucoside product entered the hydrophobic continuous phase (octanol). The surfactant clusters were enlarged during the reaction and formed inverse micelles with alcohol as cosurfactant (Fig. 8) [32].
Cluster effect of water capture; proposed micelle structure (below) [32]
This effect was also noticed by Gang et al. in solvent-free esterification in a micellar system using surfactant combined catalysts [33].
GC–MS analysis of the synthesized products of C8, C10 and C12 alcohols with glucose revealed that no oligomers or furanosides were formed. Only α- and β-anomers of glucopyranoside were found. The anomeric ratios are presented in Table 19 [32].
The anomeric ratios found were between 1.5:1 and 2:1, with α-glucopyranosides being the predominant anomer.
2.11 Sulfuric Acid and Trimethylsilyl Trifluoromethanesulfonate for Synthesis of Bromoalkyl Glycosides
Williams et al. used sulfuric acid and trimethylsilyl trifluoromethanesulfonate (TMSOTf) as catalysts for Fischer glycosylation of bromoalkyl glycosides synthesized from four different bromoalcohols and four different sugars. Sulfuric acid or TMSOTf was added to a suspension of monosaccharides and neat bromoalcohol, and the mixture was stirred at 80 °C for 12–16 h. The crude product was then purified by column chromatography, and the anomeric ratios were obtained by 1H and 13C NMR spectroscopy. The synthetic concept is shown in Fig. 9 [34].
Every sugar (d-glucose, N-acetylglucosamine, d-mannose, and d-galactose) was combined with every alcohol (bromopropanol, bromohexanol, bromooctanol, and bromodecanol), and every reaction was catalyzed with sulfuric acid or TMSOTf. The bromoalcohols were used as both solvent and reagent. The results indicated that 6 equiv. bromoalcohol relative to the monosaccharide was the lowest amount necessary to perform the glycosylation reaction. Results of the experiments are presented in Table 20 [34].
The results of the reactions with d-galactose are inaccurate because column chromatography did not result in sufficiently pure product. With the other monosaccharides, yields ranging between 5% and 86% have been shown to be possible. Only pyranosides were detected by NMR analysis, and the reactions with N-acetylglucosamine and d-mannose resulted in pure α-anomers as products. The faster a monosaccharide dissolves in the alcohol used, the higher the yield of the product. Three combinations of monosaccharide, alcohol, and catalyst were reacted with higher equivalents of alcohol to further validate this observation (Table 21) [34].
Increasing alcohol equivalents resulted in large increases of yield. Excess alcohol, which was recovered in the following column chromatography, was sufficiently pure to be reused. This legitimates the use of higher amounts of excess alcohol to increase yields [34].
3 Assisted Fischer Glycosylation
3.1 Microwave-Assisted Glycosylation
The use of microwaves (MW) has become more prevalent in modern organic chemistry. Since the first reports on the use of MW, there have been a substantial number of reports about beneficial results in organic reactions run under MW conditions compared with conventional heating [35,36,37,38]. Some of these reported benefits are faster reaction times, higher yields, and higher selectivity. Additionally, MW reactions are seen as safer and more efficient than their conventional counterparts and are therefore often a great opportunity in green chemistry. Another advantage comes through the localized heating effects of microwaves, which allows reactions with less, safer, or no solvent and helps to prevent wall effects. The efficiency of reactions run under MW conditions is often dependent on the duration and intensity of the introduced MW energy [39,40,41]. In the context of Fischer glycosylation, there are several reports in which microwaves were used to enhance the course of the reaction (Table 22).
Roy et al. used MW-assisted Fischer glycosylation to evaluate montmorillonite K-10 (MK10) as a reusable catalyst for Fischer glycosylation. In an initial experiment, the group reacted d-glucose with MK10 and methanol under conventional or MW conditions. Both approaches were optimized towards better α:β ratios through the study of different reaction times. For the conventional approach, complete conversion of the starting material was reached after 5 h, with the best results after 10 h with an α:β ratio of 13.3:1 and yield of 84%. The MW approach was run at 90 °C and provided a better α:β ratio of 14.6:1 as well as a slightly higher yield of 86%. The biggest difference was found in the optimal reaction time, which was only 10 min under MW conditions. To additionally validate the method of using MK10 with MW irradiation, the reaction was run with a number of different monosaccharides and alcohols, resulting in similarly good yields and anomeric selectivity. Selected results are presented in Table 23 [42].
To validate MK10 as a reusable catalyst, it was reused three times for glycosylation of d-glucose with benzyl alcohol. After each reaction, the MK10 was filtrated off then activated at 110 °C, with each run resulting in similar yields and the same anomeric selectivity (Table 24).
The group concluded that the use of MK10 in combination with MW irradiation represents a fast, inexpensive, and ecofriendly method for Fischer glycosylation [42].
In a study with the aim of generating simple model glycosides for lectin binding studies, Artner et al. synthesized l-glycero-α-d-manno-heptopyranoside through Fischer glycosylation of heptopyranose with methanol in presence of Dowex 50 H+ cation-exchange resin, first under classical conditions at 60 °C and second under microwave irradiation (Fig. 10) [43].
Comparatively, the conventional procedure afforded a 81% combined yield with an α-to-β ratio of 7:1 of 51 after several hours of reaction. On the other hand, microwave irradiation at 100 °C for 25 min resulted in a higher yield of 94% and an α-to-β ratio of 10:1, with the mixture additionally containing 4% of anomeric furanosides. The products were later acetylated for purification (Fig. 11) [43].
In another MW approach for Fischer glycosylation, Fröhlich et al. described the synthesis of a glucopyranoside containing a trifluoromethyl- and a thiol group for 19F NMR spectroscopy on cysteine in proteins, the 19F being necessary for NMR spectroscopy and the thiol group for selective binding to the cysteine. Only the attachment of 2,2,2-trifluoroethanol (TFE) was achieved through MW-assisted Fischer glycosylation in this research and is thus the only reaction step considered in detail here (Fig. 14). First attempts to react TFE with d-glucose under classic Fischer glycosylation conditions were unsuccessful, so the group opted for MW-assisted Fischer glycosylation under increased pressure. The group used d-glucose (1.35 g, 7.5 mmol) dissolved in TFE (15 mL) with acetic chloride (10 μL, 0.14 mmol) in a microwave reactor (Anton Paar) for 10 min at 160 °C with stirring at 600 rpm (Fig. 11a). This resulted in a conversion to the expected trifluoroethylglucoside with the pyranosides constituting the major product (75%), in which the α-anomer was the predominant anomer at 60%. In subsequent reaction steps, the group was unable to isolate the α-anomer, thus the β-anomer was used for subsequent steps, meaning the conversion of the primary hydroxy group with 2,4,6-trisisopropyl-benzenesulfonic chloride (Fig. 14b). For the later displacement of leaving group on carbon 6, the product had to be acetylated and later deprotected 57β (Fig. 14c) [44].
Ceron-Camacho et al. conducted a study to directly compare Fischer glycosylation assisted by MW versus conventional heating (CH) by synthesizing APGs from d-glucose and d-mannose with alkyl alcohols of different length. Under MW conditions, the reaction parameters were optimized through a model reaction between glucose and 1-dodecanol. The best results were obtained with a 3 min reaction time at 70 °C and 5 W of power. The same model reaction was run under CH in an oil bath at 70 °C, as well. To provide better comparability, both reactions were run in the same sealed vessel system. The group found that the MW reaction resulted in a lower conversion of glucose at 8.5% residual glucose compared with 1.4% residual glucose under CH. Also a slightly higher degree of polymerization (DP) was found under CH (DP = 1.8) compared with MW (DP = 1.6). Note that the group compared the results of both approaches in a time frame of 3 min at 70 °C, but the conventional heating took much longer at 650 s compared with 80 s for the MW to reach said temperature. This results in a higher overall reaction time for the CH [45].
In a further study, Aronow et al. questioned the scalability of MW-assisted Fischer glycosylation and thus investigated the possibility of translation into a continuous flow process, directly comparing it with the MW-mediated process. For this, see Sect. 4 [46].
3.2 Ultrasonic-Assisted Glycosylation
In the late 1920s, Loomis was the first to describe the benefits of using ultrasound in chemistry. He demonstrated an accelerating effect on the hydrolysis of dimethyl sulfate when using sound wave radiation [47]. In recent years, with the need for greener and safer approaches to organic chemistry, the use of ultrasonic waves has become a promising technique. This approach has since been incorporated into a wide variety of reactions, forming its own discipline called sonochemistry. Most sonochemical reactions are run at frequencies from 20 to 100 kHz and are driven by the kinetic energy caused through cavitation [48,49]. Cavitation describes the periodic growth and compression of air microbubbles in a liquid phase. This subsequently results in the collapse of the microbubbles, causing a rise of pressure and temperature that has been calculated to be up to 1000 atm and 500 K, respectively [50]. This results in a series of chemical and physical effects that can benefit a chemical reaction.
Amaniampong et al. reported on the possible usage of ultrasonic-assisted, catalyst-free Fischer glycosylation for production of APGs. In the current state of the art, APGs are produced by traditional acid-catalyzed Fischer glycosylation [51]. One limitation of this procedure is the attainable DP of only 1.1–1.5 glucose units per alkyl chain. A higher DP of around 2–8 would be highly beneficial for the use of APGs by enabling a number of beneficial effects [52].
In 2011, Shaik et al. proposed a procedure for improved synthesis of azidoethyl and propargyl glycopyranosides through Fischer glycosylation using H2SO4-silica (prepared according to [13]) as catalyst in addition to ultrasound radiation. The free sugars used were N-acetyl-d-glucosamine, N-acetyl-d-galactosamine, d-glucose, d-galactose, d-mannose, l-fucose, and lactose. They were glycosylated with propargyl alcohol or 2-azidoethanol at 40 °C with H2SO4-silica under ultrasonication, which was run until the starting materials disappeared. Selected results are presented in Table 24. The group noted that the success of the procedure depends on the solubility of the sugar in the used alcohol. This was seen as a possible explanation for the longer reaction time with lactose, where there was no significant improvement in reaction time compared with already existing methodology, but better yields were observed. Overall reaction times ranged from 15 min to 2 h, with the exception of lactose of 12 h. Yields also saw an improvement compared with reported procedures, ranging from 70% to 98%, and α-glycopyranosides were found to be the most common product. The group concluded that ultrasonic-assisted Fischer glycosylation is an efficient tool for glycosylation of significant monosaccharides because of its better yields and reaction times compared with traditional, Fischer glycosylation [53].
To test the usability of ultrasonic-assisted, catalyst-free Fischer glycosylation, the group subjected the sugars mannose, glucose, and xylose to ultrasonic irradiation at 550 kHz for 3 h in a solution of methanol. The impact of experimental parameters was examined by using mannose under an array of different conditions. The parameters that were modified included temperature (40 °C, 20 °C, and 0 °C at 40 wt.% man. under air), wt.% mannose (40% and 80% at 40 °C under air), and type of gas (air, Ar, and O2 at 40 °C with 40 wt.% mannose) (Table 25) [54].
It is notable that varying the temperature had no effect on reaction selectivity and resulted in a decrease in the percentage of converted mannose. The usage of Ar and O2 gases similarly reduced the conversion, to 70% and 80%. Running the reaction at 40 wt.% under air at 40 °C led to an 81% conversion with yield of 37% methyl mannosides (MeMan) and 42% Me-alkylpolymannoside with a DP higher than 2. The reaction delivered a DP range of 1–12 with an average DP of 7. The pyranoside-to-furanoside ratio was found to be 7:1, with the product consisting of 58% monosaccharides (mannose, methyl-mannosides, 1,6-anhydromannose), 12% disaccharides (Me(Man)2, Man2), and 30% APGs with DPs higher 3. Furthermore, the group was able to increase the concentration of mannose to 80 wt.% (Table 25, entry 6), resulting in a mannose conversion of 73% with APGs with DPs from 2–7 at 66% yield. The space–time yield in this scenario was found to an unprecedented 876 kg m−3 h−1 [54].
To further investigate the usability of ultrasound technology, glucose and xylose were also assessed under standard conditions (550 kHz under methanol for 3 h/6 h). After 3 h, 40% conversion of glucose was reached. By increasing the reaction time to 6 h, the conversion could be improved to 82%. For xylose, the conversion increased from an initial 82% at 3 h to 86% at 6 h. Analysis of the glucose reaction indicated an average DP of 2, with glucopyranosides being the major component at 99%. With xylose, an average DP of 3 was found with an xylopyranoside-to-xylofuranoside ratio of 1:10. Additionally, other alcohols (ethanol, n-propanol, and n-butanol) were tested, showing conversions of 70%, 66%, and 87%, respectively. Also, data obtained from matrix-assisted laser desorption–ionization (MALDI)-time of flight (TOF) analysis of the mannosidic-derived APGs revealed 1,6-anhydromannose and its mannosylated derivatives as possible key intermediates in this process.
The group concluded that high-frequency ultrasound allows selective activation (with no product having a methoxy group at a position other than the anomeric center) of the anomeric position of the analyzed sugars without the use of a catalyst as in traditional Fischer glycosylation [54].
4 Fischer Glycosylation in Reactors
One limiting factor of MW-assisted glycosylation is its poor scalability [55]. Aronow et al. proposed a transition from microwave batch to continuous flow processing for the synthesis of methyl glycosides. Preceding their proposition to use flow reactors as a possible solution to the scalability issue of MW, the group had already successfully used a continuous flow process in their report on the synthesis of l-glycero-d-manno-heptopyranose peracetate, a major constituent in Gram-negative bacteria [56]. In their proposed process, the group used a continuous flow process to glycosylate l-glycero-d-manno-heptopyranose with MeOH and sulfonic acid beads. Optimal results were found with temperatures of 90–100 °C and residence times from 10 to 25 min. The reaction resulted in a α:β pyranoside ratio of 7:1 with approximately 3.5% of α-furanoside. The methyl heptosides were then converted to the desired product in subsequent reaction steps (Fig. 12) [56].
Building on this successful use of flow chemistry, Aronow et al. conducted a comprehensive study comparing microwave and flow approaches for Fischer glycosylation. The group compared the glycosylation reactions of different sugars with methanol under the same reaction conditions in a MW-assisted approach as wells as in a flow reactor. The flow process was optimized by varying the temperature and residence time using the glycosylation reaction of d-mannose and methanol. Optimal conditions were found at 120 °C with a residence time of 4 min. These parameters were then also applied to all following MW reactions. Throughout the following reactions, high consistency between MW and flow conditions was obtained. Exemplary results of this study are presented in Table 26. Note that, for sugars that are unsolvable in MeOH, minimal amounts of water were added under flow conditions [46].
Scalability of the approach was successfully demonstrated through the formation of methyl mannosides under these standard conditions, generating a throughput of 1.2 g/h of crude product in a 10 h run. Throughout the run, a consistent α:β ratio was observed, which confirmed stable catalyst activity. After recrystallization of the crude product, the group was able to yield 9.4 g of the desired product, almost four times the mass of catalyst used [46].
Masui et al. reported on a procedure for kinetically controlled Fischer glycosylation under flow conditions for synthesis of furanosides. The approach was first tested with glucose and methanol and β-hydroxy-substituted sulfonic acid functionalized silica (HO-SAS) as catalyst. Good conditions were found to be 80 °C with 5 min residence time, resulting in 71% yield of furanosides (α:β ratio of 35:65) and 100 °C with 1 min residence time resulting in 70% yield of furanosides (α:β ratio of 43:57). Other catalysts, HCl and TsOH, were also tested, but sHO-SAS was found to be the better choice for this approach. Scalabillity and reusabillity of HO-SAS were also tested with positive results. The kinetically controlled flow approach was then applied and optimized on three additional saccharides (mannose, galactose, and N-acetylglucosamine). N-Acetylglucosamine was the only sugar for which only pyranosides were formed while no furanosides could be obtained. For mannose, the optimal conditions were found to be 100 °C with residence time of 30 s, resulting in a yield of 67% with an α:β ratio of 76:24. The optimal residence time for galactose was found to be much higher at 15 min with a temperature of 60 °C, resulting in 86% yield with an α:β ratio of 26:74 [57].
Another approach to overcome the scalability problem of MW-assisted glycosylation is the use of microreactor technology, which can be described as a scale-down technique using a device with submillimeter dimensions in which chemical reactions are performed continuously [58]. Jung et al. developed a method for Fischer glycosylation of bromoalkyl glycosides in a microreactor, which offers excellent heat and mass transfer to optimize the yield and selectivity of a reaction in a time- and material-saving manner. Microreactors are also a safer alternative for MW Fischer glycosylation, where a headspace is created through flammable alcohols, which represents an explosion hazard. Figure 13 shows the synthesis of bromoalkyl glucosides from d-glucose and bromoalcohols promoted by an acidic catalyst [59].
Figure 14 shows the process for obtaining the bromoalkyl glucosides. Dimethyl sulfoxide ((CH3)2SO) (DMSO) was used for conditioning of the microreactor and as a solvent. After stopping the reaction by neutralizing the acidic catalyst with triethylamine (Et3N), the reactor was dried with air. The crude product was purified first with column chromatography, and after combining the fractions containing bromoalkyl glucosides, organic solvents were removed under reduced pressure to allow further refinement of the product with lyophilization. Anomeric ratios were detected with 1H and 13C NMR spectroscopy [59].
The reaction was carried out with alcohols of different length and different catalysts at 75 °C, 90 °C, or 120 °C, and purification of the product was carried out by TLC, liquid–liquid extraction, and normal-phase (NP) and reverse-phase (RP) flash chromatography.
Using 2-bromoethanol or 3-bromo-1-propanol in the reaction did not produce the desired bromoalkyl glucosides. The authors assume that these short alcohols are too reactive due to the higher electrophilicity of the carbon bearing the bromide, which leads to polymeric ethyleneglycol or propyleneglycol side products. It is further discussed that potentially produced glycosidic products can react further with these side products in the microreactor or with the silica during the NP chromatographic work-up. Experiment number 3 and 4 support this assumption. In experiment 5, RP flash chromatography was used, but the reaction was carried out at 90 °C, which indicates that higher temperatures were needed to produce bromoalkyl glucosides. No product was isolated in the experiments with n-octanol when NP flash chromatography or liquid–liquid extraction was used to purify the product; only RP flash chromatography led to successful isolation of the product. Sulfuric acid (H2SO4), trifluoroacetic acid (CF3CO2H), and TMSOTf were compared in their catalytic activity with TLC. Using similar amount of reaction solution and n-octanol resulted in spots with the same RF value, but the spots were more intense when TMSOTf was used as catalyst. The authors assume that product was produced with all catalysts, but TMSOTf produced the highest yield. In the experiments where bromoalkyl glucosides were detected, TMSOTf was used as catalyst and RP flash chromatography was used as purification method. Yields between 24% and 40% were achieved with 6-bromo-1-hexanol, 8-bromo-1-octanol, and 10-bromo-1-decanol, and corresponding dibromides were isolated, which very likely are impurities in the bromoalcohols. Compared with the yields obtained by Williams et al. [34], an increase of 150% in experiments with 8-bromo-1-octanol and an increase of over 180% in experiments with 10-bromo-1-decanol were achieved. The research group points out that loss of reaction solution that occurred while handling the microreactor during the reaction is not included in the yield calculations. Since the volume of reaction solution was only 3–4 mL, even small losses of solution could significantly influence the product yield, which may be the reason for the lower yield in experiments with 6-bromo-1-hexanol compared with Williams et al. [34]. Higher yields could be expected when more reaction solution is used, since the loss of a few hundred microliters would not impact the yield calculation as much [59].
5 Outlook and Conclusions
Several advances have been made to improve the classic Fischer glycosylation for production of glycosides. New catalysts have been selected for not only their ability to increase the efficiency of their respective reaction but also being environment friendly, which is an important factor for discoveries in modern times. Protection of non-anomeric OH-groups was not necessary with most catalysts, thereby reducing reaction and purification steps. Creating a biphasic solution to capture water molecules (that result as byproduct in Fischer glycosylation) in micelles is a novel method to improve the poor solubility of alkyl glucosides in alcohol and could be explored further in the future with other monosaccharides. Microwave- and ultrasonic-assisted reactions are promising instrumental methods for Fischer glycosylation. Sonochemistry allows for much longer alkyl chains in alkyl glycosides, while microwave-assisted reactions resolve significantly faster than the standard procedure, often needing only minutes instead of hour-long reactions while retaining good yields and selectivity. However, to date, microwave-assisted glycosylation remains impractical for industrial use as it is hard to scale up. Fischer glycosylation in micro- and continuous flow reactors are relatively new processes that promise good scalability and efficient optimization of reactions. These new approaches provide good alternatives to inefficient, time-consuming, and polluting procedures for the production of glycosides.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Code availability is not applicable.
References
Berg JM (2019) Biochemistry, 9th edn. Macmillan Learning, New York
Koenigs W, Knorr E (1901) Ueber einige derivate des traubenzuckers und der Galactose. Ber Dtsch Chem Ges 34:957–981. https://doi.org/10.1002/cber.190103401162
Mydock LK, Demchenko AV (2010) Mechanism of chemical O-glycosylation: from early studies to recent discoveries. Org Biomol Chem 8:497–510. https://doi.org/10.1039/b916088d
Das R, Mukhopadhyay B (2016) Chemical O-glycosylations: an overview. ChemistryOpen 5:401–433. https://doi.org/10.1002/open.201600043
Schmidt RR (1986) New methods for the synthesis of glycosides and oligosaccharides? Are there alternatives to the Koenigs–Knorr method? [New Synthetic Methods (56)]. Angew Chem Int Ed Engl 25:212–235. https://doi.org/10.1002/anie.198602121
Paulsen H (1982) Advances in selective chemical syntheses of complex oligosaccharides. Angew Chem Int Ed Engl 21:155–173. https://doi.org/10.1002/anie.198201553
Nielsen MM, Pedersen CM (2018) Catalytic glycosylations in oligosaccharide synthesis. Chem Rev 118:8285–8358. https://doi.org/10.1021/acs.chemrev.8b00144
Andreana PR, Crich D (2021) Guidelines for O-glycoside formation from first principles. ACS Cent Sci 7:1454–1462. https://doi.org/10.1021/acscentsci.1c00594
Fischer E (1893) Ueber die Glucoside der Alkohole. Ber Dtsch Chem Ges 26:2400–2412. https://doi.org/10.1002/cber.18930260327
Lindhorst TK (2007) Essential of carbohydrate chemistry and biochemistry: with 150 new exercises, 3., completely rev. and enl. Wiley-VCH, Weinheim
Velty R, Benvegnu T, Gelin M et al (1997) A convenient synthesis of disaccharides containing furanoside units. Carbohydr Res 299:7–14. https://doi.org/10.1016/S0008-6215(96)00268-6
Aich U, Loganathan D (2007) Zeolite-catalyzed Helferich-type glycosylation of long-chain alcohols. Synthesis of acetylated alkyl 1,2-trans glycopyranosides and alkyl 1,2-cis C2-hydroxy-glycopyranosides. Carbohydr Res 342:704–709. https://doi.org/10.1016/j.carres.2006.12.014
Roy B, Mukhopadhyay B (2007) Sulfuric acid immobilized on silica: an excellent catalyst for Fischer type glycosylation. Tetrahedron Lett 48:3783–3787. https://doi.org/10.1016/j.tetlet.2007.03.165
(2002) Zeolites for cleaner technologies: lectures presented at the Poitiers school—the meeting Zeolites for Cleaner Technologies, a pre-conference school held in early July 2001 in Poitiers and organized before the 13th International Zeolite Conference (Montpellier, July 2001). Catalytic science series, vol 3. ICP Imperial College Press, London
Rauter AP, Almeida T, Xavier NM et al (2007) Acid zeolites as efficient catalysts for O- and S-glycosylation. J Mol Catal A Chem 275:206–213. https://doi.org/10.1016/j.molcata.2007.06.002
Augé J, Sizun G (2009) Ionic liquid promoted atom economic glycosylation under Lewis acid catalysis. Green Chem 11:1179. https://doi.org/10.1039/b904692e
Guchhait G, Misra AK (2011) Efficient glycosylation of unprotected sugars using sulfamic acid: a mild eco-friendly catalyst. Catal Commun 14:52–57. https://doi.org/10.1016/j.catcom.2011.07.016
Ajisaka K, Yagura M, Miyazaki T (2012) A novel two-step synthesis of α-linked mannobioses based on an acid-assisted reverse hydrolysis reaction. Carbohydr Res 347:147–150. https://doi.org/10.1016/j.carres.2011.10.037
van Es DS, Marinkovic S, Oduber X et al (2013) Use of furandicarboxylic acid and its decyl ester as additives in the Fischer’s glycosylation of decanol by d-glucose: physicochemical properties of the surfactant compositions obtained. J Surfact Deterg 16:147–154. https://doi.org/10.1007/s11743-012-1382-8
Sharma DK, Lambu MR, Sidiq T et al (2013) Ammonium chloride mediated synthesis of alkyl glycosides and evaluation of their immunomodulatory activity. RSC Adv 3:11450. https://doi.org/10.1039/C3RA41050A
Mohan R (2010) Green bismuth. Nat Chem 2:336. https://doi.org/10.1038/nchem.609
Polanki IK, Kurma SH, Bhattacharya AK (2015) Direct glycosylation of unprotected and unactivated sugars using bismuth nitrate pentahydrate. J Carbohydr Chem 34:196–205. https://doi.org/10.1080/07328303.2015.1028585
Wessel HP (1988) Use of trifluoromethanesulfonic acid in Fischer glycosylations. J Carbohydr Chem 7:263–269. https://doi.org/10.1080/07328308808058919
Manhas S, Taylor MS (2017) Boronic acids as phase-transfer reagents for Fischer glycosidations in low-polarity solvents. J Org Chem 82:11406–11417. https://doi.org/10.1021/acs.joc.7b01880
Imamura A, Lowary T (2011) Chemical synthesis of furanose glycosides. TIGG 23:134–152. https://doi.org/10.4052/tigg.23.134
Kaji E, Yamamoto D, Shirai Y et al (2014) Thermodynamically controlled regioselective glycosylation of fully unprotected sugars through bis(boronate) intermediates. Eur J Org Chem 2014:3536–3539. https://doi.org/10.1002/ejoc.201402255
Dahlhoff WV (1987) Amphiphilic carbohydrate-based mesogens; I. Mesognic O-n-alkyl β-d-mannofuranosides: synthesis of a novel homologous series of glycosides. Synthesis 1987:366–368. https://doi.org/10.1055/s-1987-27945
Willför S, Sundberg K, Tenkanen M et al (2008) Spruce-derived mannans—a potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr Polym 72:197–210. https://doi.org/10.1016/j.carbpol.2007.08.006
Vashishtha M, Mishra M, Undre S et al (2015) Molecular mechanism of micellar catalysis of cross aldol reaction: effect of surfactant chain length and surfactant concentration. J Mol Catal A Chem 396:143–154. https://doi.org/10.1016/j.molcata.2014.09.023
Manabe K, Sun XM, Kobayashi S (2001) Dehydration reactions in water. Surfactant-type Brønsted acid-catalyzed direct esterification of carboxylic acids with alcohols in an emulsion system. J Am Chem Soc 123:10101–10102. https://doi.org/10.1021/ja016338q
Stamatis H, Xenakis A, Menge U et al (1993) Kinetic study of lipase catalyzed esterification reactions in water-in-oil microemulsions. Biotechnol Bioeng 42:931–937. https://doi.org/10.1002/bit.260420803
Nowicki J, Woch J, Mościpan M et al (2017) Micellar effect on the direct Fischer synthesis of alkyl glucosides. Appl Catal A-Gen 539:13–18. https://doi.org/10.1016/j.apcata.2017.04.002
Gang L, Xinzong L, Eli W (2007) Solvent-free esterification catalyzed by surfactant-combined catalysts at room temperature. New J Chem 31:348. https://doi.org/10.1039/b615448d
Williams L, Schunck N, Götz KH et al (2019) Systematic synthesis and characterization of a series of different bromoalkylglycosides by Fischer glycosylation. Carbohydr Res 486:107841. https://doi.org/10.1016/j.carres.2019.107841
Gedye R, Smith F, Westaway K et al (1986) The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett 27:279–282. https://doi.org/10.1016/S0040-4039(00)83996-9
Giguere RJ, Bray TL, Duncan SM et al (1986) Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett 27:4945–4948. https://doi.org/10.1016/S0040-4039(00)85103-5
Bornaghi LF, Poulsen S-A (2005) Microwave-accelerated Fischer glycosylation. Tetrahedron Lett 46:3485–3488. https://doi.org/10.1016/j.tetlet.2005.03.126
Beckmann HSG, Wittmann V (2007) One-pot procedure for diazo transfer and azide-alkyne cycloaddition: triazole linkages from amines. Org Lett 9:1–4. https://doi.org/10.1021/ol0621506
Bassyouni FA, Abu-Bakr SM, Rehim MA (2012) Evolution of microwave irradiation and its application in green chemistry and biosciences. Res Chem Intermed 38:283–322. https://doi.org/10.1007/s11164-011-0348-1
Egami H, Hamashima Y (2019) Practical and scalable organic reactions with flow microwave apparatus. Chem Rec 19:157–171. https://doi.org/10.1002/tcr.201800132
Martina K, Cravotto G, Varma RS (2021) Impact of microwaves on organic synthesis and strategies toward flow processes and scaling up. J Org Chem 86:13857–13872. https://doi.org/10.1021/acs.joc.1c00865
Roy DK, Bordoloi M (2008) Montmorillonite K-10 as a reusable catalyst for Fischer type of glycosylation under microwave irradiation. J Carbohydr Chem 27:300–307. https://doi.org/10.1080/07328300802107437
Artner D, Stanetty C, Mereiter K et al (2011) Crystal and molecular structure of methyl L-glycero-α-D-manno-heptopyranoside, and synthesis of 1→7 linked L-glycero-D-manno-heptobiose and its methyl α-glycoside. Carbohydr Res 346:1739–1746. https://doi.org/10.1016/j.carres.2011.05.033
Fröhlich RFG, Schrank E, Zangger K (2012) 2,2,2-Trifluoroethyl 6-thio-β-D-glucopyranoside as a selective tag for cysteines in proteins. Carbohydr Res 361:100–104. https://doi.org/10.1016/j.carres.2012.08.010
Cerón-Camacho R, Aburto JA, Montiel LE et al (2013) Microwave-assisted organic synthesis versus conventional heating. A comparative study for Fisher glycosidation of monosaccharides. C R Chim 16:427–432. https://doi.org/10.1016/j.crci.2012.12.011
Aronow J, Stanetty C, Baxendale IR et al (2019) Methyl glycosides via Fischer glycosylation: translation from batch microwave to continuous flow processing. Monatsh Chem 150:11–19. https://doi.org/10.1007/s00706-018-2306-8
Richards WT, Loomis AL (1927) The chemical effects of high frequency sound waves I. A preliminary survey. J Am Chem Soc 49:3086–3100. https://doi.org/10.1021/ja01411a015
Martínez RF, Cravotto G, Cintas P (2021) Organic sonochemistry: a chemist’s timely perspective on mechanisms and reactivity. J Org Chem 86:13833–13856. https://doi.org/10.1021/acs.joc.1c00805
Bera S, Mondal D, Martin JT et al (2015) Potential effect of ultrasound on carbohydrates. Carbohydr Res 410:15–35. https://doi.org/10.1016/j.carres.2015.02.008
Pokhrel N, Vabbina PK, Pala N (2016) Sonochemistry: science and engineering. Ultrason Sonochem 29:104–128. https://doi.org/10.1016/j.ultsonch.2015.07.023
von Rybinski W, Hill K (1998) Alkyl polyglycosides—properties and applications of a new class of surfactants. Angew Chem Int Ed Engl 37:1328–1345. https://doi.org/10.1002/(SICI)1521-3773(19980605)37:10%3c1328:AID-ANIE1328%3e3.0.CO;2-9
Ericsson CA, Ericsson LC, Kocherbitov V et al (2005) Thermotropic phase behaviour of long-chain alkylmaltosides. Phys Chem Chem Phys 7:2970–2977. https://doi.org/10.1039/b502922h
Shaikh N, Russo L, Cipolla L et al (2011) Ultrasonic assisted Fischer glycosylation: generating diversity for glycochemistry. Mol Divers 15:341–345. https://doi.org/10.1007/s11030-010-9281-2
Amaniampong PN, Clément J-L, Gigmes D et al (2018) Catalyst-free synthesis of alkylpolyglycosides induced by high-frequency ultrasound. Chemsuschem 11:2673–2676. https://doi.org/10.1002/cssc.201801137
Glasnov TN, Kappe CO (2011) The microwave-to-flow paradigm: translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chemistry 17:11956–11968. https://doi.org/10.1002/chem.201102065
Kosma P (2008) Occurrence, synthesis and biosynthesis of bacterial heptoses. COC 12:1021–1039. https://doi.org/10.2174/138527208785161169
Masui S, Manabe Y, Hirao K et al (2019) Kinetically controlled Fischer glycosidation under flow conditions: a new method for preparing furanosides. Synlett 30:397–400. https://doi.org/10.1055/s-0037-1611643
Watts P, Wiles C (2007) Recent advances in synthetic micro reaction technology. Chem Commun (Camb) 25:443–467. https://doi.org/10.1039/b609428g
Jung J, Kaiser L, Deigner H-P et al (2021) Continuous synthesis of bromoalkyl glycosides by Fischer glycosylation in a microreactor. J Flow Chem. https://doi.org/10.1007/s41981-021-00202-0
Acknowledgements
We thank the ReAching program of the faculty MLS, Furtwangen University, for support.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
K.W. and M.H. performed the literature search. The manuscript was written by .K.W and M.H. The tables and images were produced by K.W. and M.H. The complete writing, design, and corrections of the manuscript were led and supervised by J.J. and M.S.S. All authors read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial or nonfinancial interest in the subject matter or materials discussed in this manuscript. All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Participant consent is not applicable.
Consent for publication
Publication consent is not applicable.
Informed consent
Informed consent is not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haese, M., Winterhalter, K., Jung, J. et al. Like Visiting an Old Friend: Fischer Glycosylation in the Twenty-First Century: Modern Methods and Techniques. Top Curr Chem (Z) 380, 26 (2022). https://doi.org/10.1007/s41061-022-00383-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-022-00383-9