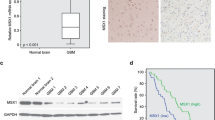
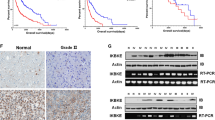
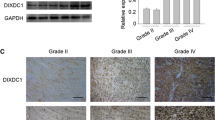
Abstract
Glioblastoma is the most common type of astrocytoma in the brain. Due to its high invasiveness and chemoresistance, patients with advanced stage of glioblastoma have a poor prognosis. SNAI1, an important regulator of epithelial-mesenchymal transition, has been associated with metastasis in various carcinoma cells. However, its roles in glioblastoma cells have been poorly characterized. To examine roles of SNAI1 in glioblastoma cells, we knockdowned SNAI1 expression using siRNA. SNAI1 siRNA increased the expression level of E-cadherin and decreased that of vimentin. In the water-soluble tetrazolium salt (WST-1) assay, SNAI1 siRNA inhibited the proliferation of U87-MG and GBM05 glioblastoma cells. Moreover, in the Boyden chamber assay and Matrigel invasion assay, SNAI1 siRNA inhibited serum-induced migration and invasion of glioblastoma cells. These results suggested that SNAI1 is involved in the proliferation and migration of glioblastoma cells.





Similar content being viewed by others
References
Barrallo-Gimeno A, Nieto MA (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132:3151–3161
Birchmeier W, Behrens J (1994) Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1198:11–26
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21:3241–3246
Boulay JL, Dennefeld C, Alberga A (1987) The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature 330:395–398
Choudhary R, Li H, Winn RA, Sorenson AL, Weiser-Evans MC, Nemenoff RA (2010) Peroxisome proliferator-activated receptor-gamma inhibits transformed growth of non-small cell lung cancer cells through selective suppression of Snail. Neoplasia 12:224–234
Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103:1631–1643
Emadi Baygi M, Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W, Schulz WA (2010) Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines. Tumour Biol 31:297–307
Haraguchi M, Okubo T, Miyashita Y, Miyamoto Y, Hayashi M, Crotti TN, McHugh KP, Ozawa M (2008) Snail regulates cell-matrix adhesion by regulation of the expression of integrins and basement membrane proteins. J Biol Chem 283:23514–23523
Hemavathy K, Ashraf SI, Ip YT (2000) Snail/slug family of repressors: slowly going into the fast lane of development and cancer. Gene 257:1–12
Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C (2009) Glioblastoma multiforme oncogenomics and signaling pathways. Clin Med Oncol 3:39–52
Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA (2009) Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells 27:2059–2068
Liu J, Uygur B, Zhang Z, Shao L, Romero D, Vary C, Ding Q, Wu WS (2010) Slug inhibits proliferation of human prostate cancer cells via downregulation of cyclin D1 expression. Prostate 70:1768–1777
Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K, Miyazaki K (2005) Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer 92:252–258
Motta FJ, Valera ET, Lucio-Eterovic AK, Queiroz RG, Neder L, Scrideli CA, Machado HR, Carlotti-Junior CG, Marie SK, Tone LG (2008) Differential expression of E-cadherin gene in human neuroepithelial tumors. Genet Mol Res 7:295–304
Myung J, Cho BK, Kim YS, Park SH (2010) Snail and Cox-2 expressions are associated with WHO tumor grade and survival rate of patients with gliomas. Neuropathology 30:224–231
Nieto MA (2002) The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 3:155–166
Nieto MA, Sargent MG, Wilkinson DG, Cooke J (1994) Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science 264:835–839
Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, de la Fuente R, Bernad A, Pollan M, Bonilla F, Gamallo C, de Herreros AG, Munoz A (2004) The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med 10:917–919
Peinado H, Ballestar E, Esteller M, Cano A (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24:306–319
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Pena C, Garcia JM, Silva J, Garcia V, Rodriguez R, Alonso I, Millan I, Salas C, de Herreros AG, Munoz A, Bonilla F (2005) E-cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet 14:3361–3370
Rasheed BK, Wiltshire RN, Bigner SH, Bigner DD (1999) Molecular pathogenesis of malignant gliomas. Curr Opin Oncol 11:162–167
Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF (2002) Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 161:1881–1891
Sharma M, Henderson BR (2007) IQ-domain GTPase-activating protein 1 regulates beta-catenin at membrane ruffles and its role in macropinocytosis of N-cadherin and adenomatous polyposis coli. J Biol Chem 282:8545–8556
Sugimachi K, Tanaka S, Kameyama T, Taguchi K, Aishima S, Shimada M, Sugimachi K, Tsuneyoshi M (2003) Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res 9:2657–2664
Takeno S, Noguchi T, Fumoto S, Kimura Y, Shibata T, Kawahara K (2004) E-cadherin expression in patients with esophageal squamous cell carcinoma: promoter hypermethylation, snail overexpression, and clinicopathologic implications. Am J Clin Pathol 122:78–84
Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18:1131–1143
Xia M, Hu M, Wang J, Xu Y, Chen X, Ma Y, Su L (2010) Identification of the role of Smad interacting protein 1 (SIP1) in glioma. J Neurooncol 97:225–232
Yang MH, Chang SY, Chiou SH, Liu CJ, Chi CW, Chen PM, Teng SC, Wu KJ (2007) Overexpression of NBS1 induces epithelial-mesenchymal transition and co-expression of NBS1 and Snail predicts metastasis of head and neck cancer. Oncogene 26:1459–1467
Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, Peng WL, Wu JC (2009) Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology 50:1464–1474
Yang HW, Menon LG, Black PM, Carroll RS, Johnson MD (2010) SNAI2/Slug promotes growth and invasion in human gliomas. BMC Cancer 10:301
Yilmaz M, Christofori G (2009) EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev 28:15–33
Yokoyama K, Kamata N, Fujimoto R, Tsutsumi S, Tomonari M, Taki M, Hosokawa H, Nagayama M (2003) Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol 22:891–898
Acknowledgments
This study was supported by Medical Research Institute Grant (2006-12), Pusan National University and a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sung-Pil Han and Ji-Hoon Kim equally contributed to this work.
Rights and permissions
About this article
Cite this article
Han, SP., Kim, JH., Han, ME. et al. SNAI1 is Involved in the Proliferation and Migration of Glioblastoma Cells. Cell Mol Neurobiol 31, 489–496 (2011). https://doi.org/10.1007/s10571-010-9643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9643-4