Abstract
The Chromogranin A (CgA)-derived anti-hypertensive peptide catestatin (CST) antagonizes catecholamine secretion, and is a negative myocardial inotrope acting via a nitric oxide-dependent mechanism. It is not known whether CST contributes to ischemia/reperfusion injury or is a component of a cardioprotective response to limit injury. Here, we tested whether CST by virtue of its negative inotropic activity improves post-ischemic cardiac function and cardiomyocyte survival. Three groups of isolated perfused hearts from adult Wistar rats underwent 30-min ischemia and 120-min reperfusion (I/R, Group 1), or were post-conditioned by brief ischemic episodes (PostC, 5-cycles of 10-s I/R at the beginning of 120-min reperfusion, Group 2), or with exogenous CST (75 nM for 20 min, CST-Post, Group-3) at the onset of reperfusion. Perfusion pressure and left ventricular pressure (LVP) were monitored. Infarct size was evaluated with nitroblue-tetrazolium staining. The CST (5 nM) effects were also tested in simulated ischemia/reperfusion experiments on cardiomyocytes isolated from young-adult rats, evaluating cell survival with propidium iodide labeling. Infarct size was 61 ± 6% of risk area in hearts subjected to I/R only. PostC reduced infarct size to 34 ± 5%. Infarct size in CST-Post was 36 ± 3% of risk area (P < 0.05 respect to I/R). CST-Post reduced post-ischemic rise of diastolic LVP, an index of contracture, and significantly improved post-ischemic recovery of developed LVP. In isolated cardiomyocytes, CST increased the cell viability rate by about 65% after simulated ischemia/reperfusion. These results suggest a novel cardioprotective role for CST, which appears mainly due to a direct reduction of post-ischemic myocardial damages and dysfunction, rather than to an involvement of adrenergic terminals and/or endothelium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chromogranin A (CgA), a 48-kDa acidic secretory protein, is highly conserved in the vertebrate secretory granules of both the diffuse neuroendocrine system (Helle et al. 2007, Helle 2010) and the heart itself (Pieroni et al. 2007), where it is co-stored and co-secreted with catecholamines and natriuretic peptides. CgA plasma levels have long been used for clinical applications as a biomarker of neuroendocrine tumors (O’Connor et al. 2008). Recently, CgA has emerged as a marker of cardiovascular dysfunctions, such as essential hypertension (Mahapatra et al. 2005; Rao et al. 2007; Jansson et al. 2009), hypertrophic/dilatative cardiomyopathy, and heart failure (Ceconi et al. 2002; Pieroni et al. 2007). Of note, in acute coronary syndromes, circulating levels of CgA provide prognostic information independently from conventional risk markers, predicting long-term mortality and heart failure hospitalizations during follow-up (Jansson et al. 2009). Moreover, increased plasma levels of CgA are present in patients after myocardial infarction (Omland et al. 2003). In a recent study, though the circulating CgA levels are associated with several established risk markers in chronic heart failure (CHF) patients, including increased age, diabetes, reduced renal function, and heart rate variability, the CgA levels did not provide incremental prognostic information to that obtained from other established parameters (Rosjo et al. 2010).
The involvement of CgA in cardiovascular homeostasis is also strongly supported by its function as a prohormone. Via post-translational proteolytic processing, it gives rise to bioactive peptides implicated in various counter-regulatory processes (Helle et al. 2007, Helle 2010). Recently, we showed that the N-terminal CgA-derived Vasostatin 1 (VS-1; human recombinant CgA1–78) protects against the extension of myocardial infarction in the rat, inducing a pre-conditioning-like effect via adenosine/nitric oxide (NO) signaling if administered at low concentration before ischemia/reperfusion (I/R) (Cappello et al. 2007). VS-1 is also able to counteract the effects of adrenergic stimulation (Cerra et al. 2006) via an endothelial and endocardial release of nitric oxide, thus contributing to protection against excessive excitatory sympathetic challenges (Gallo et al. 2007; Cerra et al. 2008).
Among the other CgA-derived peptides, catestatin (CST, hCgA352–372) is known to exert several in vivo and in vitro cardiovascular activities. It is an endogenous non-competitive antagonist of nicotine-evoked catecholamine secretion (O’Connor and Deftos 1986; O’Connor et al. 2002; Mahata et al. 1997, 2000, 2004; Herrero et al. 2002; Mahapatra et al. 2005; Mahata et al. 2010), which induces vasodilatation through both inhibition of catecholamine release and increased circulating levels of histamine (Kennedy et al. 1998). Recent studies indicate that CST also caused vasodilation in human subjects (Fung et al. 2010). CST plasma levels are decreased not only in hypertensive patients but also in their still-normotensive offsprings (O’Connor et al. 2002). Consistent with these human studies, exogenous CST rescues arterial hypertension of CgA knockout mice (Mahapatra et al. 2005). Recently, on the isolated rat heart, CST was found to elicit, similarly to VS-1, negative inotropic and lusitropic actions, as well as a vasorelaxant influence on coronary arteries pre-contracted by either isoproterenol or endothelin-1 (Cerra et al. 2006; Angelone et al. 2008). Recently, it has been shown that CST replacement improves dampened baroreflex sensitivity (Gayen et al. 2009) and heart rate variability (Dev et al. 2010) in CgA knockout mice. Taken together, these data point to CST as a novel regulator of cardiac function and blood pressure (Mahata et al. 2010; Helle 2010).
On the basis of these cardiovascular effects of CST, the possibility exists that this CgA-derived peptide exerts cardioprotective influence under I/R conditions. Indeed, cardioprotection includes endothelial and adrenergic components (Bell and Yellon 2003; Pagliaro et al. 2003; Cappello et al. 2007; Heusch et al. 2008), which are known to be affected by CST (via anti-endothelin-1/pro-nitric oxide and anti-adrenergic actions, respectively (Mahata et al. 2000; Herrero et al. 2002; Angelone et al. 2008). This provides a rationale for the hypothesis that CST may influence the cardioprotective response. Since the role of CST in this aspect is yet to be addressed, in this study, we aimed to explore the CST involvement in cardioprotection, using both Langendorff reperfused rat heart and isolated cardiomyocytes. In particular, we tested whether CST, applied after an infarcting ischemia, could improve recovery of post-ischemic cardiac function, limiting infarct size in isolated heart. For comparative purpose, we also studied ischemic post-conditioning (PostC), which is a well-known protective procedure (Couvreur et al. 2006; Penna et al. 2006, 2008a, b, 2009a, b, c; Hausenloy et al. 2009). To exclude a role for the endothelial and neural effects, we also tested whether CST may limit cell death in a model of isolated cardiomyocytes exposed to simulated ischemia/reperfusion.
Materials and Methods
Animals
Male Wistar rats were used in accordance with Italian law (DL-116, January 27, 1992) and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Isolated Heart Perfusion
The methods were similar to those previously described (Penna et al. 2006, 2008b, c, 2009a, b, c). In brief, excised hearts were paced and constant-flow perfused with Krebs solution. Hearts were then subjected to 30-min zero-flow global ischemia, followed by 120-min reperfusion (Group 1, I/R). In a second group, hearts underwent a protocol of PostC (i.e., five cycles of 10-s reperfusion and ischemia (Penna et al. 2006, 2008b, 2009a, b, c). In Group 3 (CST-Post group, n = 7) in lieu of PostC, CST (75 nM) was infused for 20 min at the beginning of reperfusion (Fig. 1a). The concentration of CST was chosen on the basis of a preliminary dose–response curve (from 33 to 100 nM) as the dose that induced the highest infarct size reduction (data not shown).
Experimental design: a Isolated, Langendorff-perfused hearts. Hearts were stabilized for 40 min, and then subjected to 30 min of normothermic global ischemia followed by 120 min of reperfusion. Post-conditioning (PostC) protocol (5 cycles 10-s ischemia/reperfusion) is indicated by vertical lines at the beginning of reperfusion period. CST-Post group received Catestatin (CST, 75 nM) during the initial 20-min reperfusion. b Isolated cardiomyocytes. Tyrode group: cardiomyocytes were superfused/reperfused with Tyrode for 30 min. Ischemic buffer group: superfusion with Tyrode solution for 5 min, ischemic buffer for 15 min, and Tyrode (reperfusion) for 5 min. Ischemic buffer + CST group: superfusion with Tyrode + 5 nM CST for 5 min, ischemic buffer + 5 nM CST for 15 min and Tyrode alone for 5 min (reperfusion). The cellular viability was evaluated by propidium iodide (PI, 10 μg/ml) labeling. The points I, II, and III indicated by the arrows correspond to the times of image acquisition for each experimental condition (for further explanation see text)
Left ventricular pressure (LVP) was monitored throughout the experiments and infarct size was determined at the end of reperfusion.
Simulated Ischemia/Reperfusion on Isolated Adult Rat Cardiomyocytes
Isolated cardiomyocytes were obtained from the hearts of adult rats (n = 7, 200–300 g body wt) according to the previously described method (Gallo et al. 2007). Preliminary experiments were performed to determine the optimal conditions for simulating I/R. Ischemic HEPES buffer is described in “Solutions and drugs”. In the simulated I/R protocol (IB), cardiomyocytes were first superfused with oxygenated Tyrode solution for 5 min, followed by 15 min of ischemic buffer, and 5 min of Tyrode (reperfusion). In the control protocol (Ctrl), cardiomyocytes were superfused/reperfused with Tyrode for a total of 30 min. In the IB + CST protocol, cardiomyocytes were superfused with Tyrode + 5 nM CST for 5 min, ischemic buffer + 5 nM CST for 15 min, and Tyrode alone for 5 min (Fig. 1b). Cellular viability was evaluated by propidium iodide (PI, 10 μg/ml) labeling. Images were acquired using a laser scanning confocal system (Fluoview 200, Olympus America, Melville, NY) with an Ar/Kr laser (488 and 568 nm) mounted on an inverted microscope (model IX70, Olympus) equipped with a ×20 UplanApo (NA 0.90). Confocal image acquisitions for each experimental condition were performed at the times I, II, and III indicated in Fig. 1b.
Solutions and Drugs
Tyrode control solution contained (mM): 154 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5.5 d-glucose, 5 HEPES, pH 7.4 adjusted with NaOH. The Ca2+-free Tyrode solution used in cell isolation was the control Tyrode without CaCl2 and with 10-mM butanedionemonoxime (BDM, Sigma), pH adjusted with NaOH. Isolated cardiomyocytes were cultured in M1018 medium (Sigma), 1% FBS, 100-U/ml penicillin, 100-mg/ml streptomycin, 1:1,000 insulin–transferrin–selenium (ITS, Sigma), 10-mM BDM. Ischemic buffer used for I/R experiments contained (mM) 137 NaCl, 3.5 KCl, 0.88 CaCl2, 0.51 MgSO4, 5.5 d-glucose, 4 HEPES, 10 2-deoxy-d-glucose, and 20 dl-lactic acid (pH 6.5).
Statistical Analysis
All data are expressed as means ± SEM. One-way ANOVA and Newman–Keuls multiple comparison test (for post-ANOVA comparisons) have been used to compare infarct size; one-way ANOVA with the use of SNK test for post hoc analysis have been used to compare cellular viability. Functional data were compared with repeated measures ANOVA (time/group). A t test with Bonferroni correction was also used to compare the last-time points of functional data. Differences with P < 0.05 were regarded as statistically significant.
Results
Isolated Hearts
Infarct Size
The risk area, i.e., left ventricular (LV) mass, was similar in all groups (LV weight was 927 ± 14; range 559–1105 mg). Total infarct size (Fig. 2), expressed as a percentage of LV mass was 61 ± 5 in I/R (Group 1). PostC (Group 2) significantly reduced the infarct size to 34 ± 5 (P < 0.01 with respect to I/R). The infusion of CST (75 nM) during early 20-min reperfusion reduced infarct size to 42 ± 4% of LV mass (P < 0.01 with respect to the control, NS with respect to PostC).
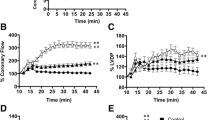
Systolic function: a Percent variation of developed LVP (dLVP) with respect to baseline level for each group, during the 30-min ischemia and 120-min reperfusion. b Percent variation of first derivative of LVP during systole (dP/dt max) with respect to baseline level for each group, during the 30-min ischemia and 120-min reperfusion. Time 0 correspond to the beginning of reperfusion. PostC post-conditioning, CST catestatin. * P < 0.05 with respect to I/R; # P < 0.05 with respect to CST-Post. Diastolic function: c Left ventricular end-diastolic pressure (LVEDP) (mmHg) during the 30-min ischemia and 120-min reperfusion. d Percent variation of first derivative of LVP during diastole (dP/dt min) with respect to baseline level for each group, during the 30-min ischemia and 120-min reperfusion Time 0 correspond to the beginning of reperfusion. PostC post-conditioning, CST catestatin. ** P < 0.01 with respect to I/R (for further explanation see text)
Cardiac Functional Parameters
Baseline values of the considered parameters are reported in Table 1.
Systolic Function
In Fig. 3a and b, developed LVP (dLVP) and maximum rate of increase of LVP during systole (dP/dt max) are reported as percent variation with respect to baseline level. The hearts of the I/R group present a marked limitation of dLVP recovery; in fact at the end of reperfusion dLVP was 34 ± 10% of baseline level (P < 0.001). PostC and CST-Post significantly improved the dLVP recovery during reperfusion (P < 0.05 with respect to I/R group, for both). Actually, the improvement observed after CST was significantly higher (P < 0.05) than that observed in PostC group. In particular, at the end of reperfusion the recovery was 52 ± 10% (P < 0.05) and 63 ± 23% (P < 0.05) of baseline levels for PostC and CST-Post, respectively (Fig. 3a). A similar trend was observed for dP/dt max recovery during reperfusion in the three groups, though statistic differences were observed with respect to I/R group only, and not between PostC and CST-Post (Fig. 3b).
Diastolic Function
Diastolic function is represented by the level of end-diastolic LVP (LVEDP) and maximum rate of decrease of LVP during diastole (dP/dt min) during ischemia and reperfusion (Fig. 3c, d). Contracture can be defined as an increase in LVEDP of 4 mmHg above the baseline level (Baker et al. 2000; Pagliaro et al. 2003). I/R markedly increased contracture. During reperfusion both PostC and CST-Post significantly limited contracture development (Fig. 3c); in fact LVEDP at the end of reperfusion was 22 ± 7 and 35 ± 7 mmHg in PostC and CST-Post, respectively (P < 0.01 with respect to I/R for both). Accordingly, dP/dt min recovery during reperfusion was significantly improved by both PostC and CST (Fig. 3d).
Isolated Adult Rat Cardiomyocytes
The protective role of CST was also investigated on isolated adult cardiomyocytes subjected to simulated I/R, as indicated in the “Methods” (Fig. 1b). Image acquisitions for each experimental condition (Ctrl, IB, or IB + CST) were performed at the times I, II, and III, showed in Fig. 1b. Representative experiments are presented in Fig. 4a. With respect to control (Ctrl), the appearance of propidium iodide staining at the end of reperfusion (III) in the ischemic (IB) sample clearly demonstrates the effectiveness of I/R simulation. CST (5 nM) administration (IB + CST) significantly (P < 0.05, Fig. 4b) preserved cell viability after reperfusion, being comparable to that observed in control condition. Figure 4b summarizes the results of these experiments: viability rate was 81.3 ± 10.8% in control, 12.9 ± 8.3% in IB, and 63.5 ± 17.0% in IB + CST.
Cardiomyocytes survival after simulated I/R: representative experiment of a simulated I/R experiment: freshly isolated cardiomyocytes adhered on glass bottom dishes were placed under the confocal microscope and processed with the in vitro ischemia/reperfusion protocol as indicated in Fig. 1b. Cell viability was assessed by monitoring the time course of propidium iodide (PI) staining. Confocal Image acquisitions for each experimental condition (Tyr, IB, or CST) were performed at the times I, II, and III indicated Fig. 1b. Cells damaged by I/R are indicated by white (pre-reperfusion) or black (post-reperfusion) arrows (for further explanation see text). Bar graph summarizes the viability rate in the control protocol (Ctrl: 81.25%, 28 cells from three different experiments), in the I/R protocol (IB: 64 cells from seven experiments), and in the catestatin protocol (IB + CST: 45 cells from six experiments). Viability rate was quantified as the number of unstained cells at the end of reperfusion (PostRep) with respect to the number of unstained cells at t = 0 (PreRep) (no cells PostRep/no cells PreRep) × 100. Results are presented as mean ± SEM. * P < 0.05
Discussion
CST (hCgA352–372; bCgA344–364), proteolytically processed from CgA, is the most potent endogenous antagonist of nicotinic-cholinergic receptor that inhibits nicotine-evoked catecholamine secretion in an autocrine/paracrine fashion (Mahata et al. 1997; 2010). It acts also as an anti-endothelin-1 and pro-nitric oxide agent ex vivo (Angelone et al. 2008) and as a potent vasodilator in vivo (Kennedy et al. 1998), mainly through stimulation of histamine release, as additionally demonstrated in vitro from mast cells (Mahata et al. 2004). These findings, together with its suggested implication as an endogenous anti-hypertensive regulator (Mahapatra et al. 2005; Rao et al. 2007), point to CST as a novel cardiac modulator to protect the heart against excessive sympatho-chromaffin over-activation that could significantly influence the onset and the course of patho-physiological conditions.
We have shown that in isolated rat hearts CST, given at reperfusion (CST-Post), decreased the infarct size, limited contracture and improved the post-ischemic systolic function. Furthermore, CST was found to be protective in a model of isolated cardiomyocytes exposed to simulated ischemia, increasing cell viability rate of about 65%. In this study, the post-ischemic rat heart was perfused with a CST concentration (75 nM), which is within the same range of concentrations of the precursor CgA, detected in plasma of patients suffering myocardial infarction (about 1 nM) or CHF (about 10 nM) (Ceconi et al. 2002; Omland et al. 2003). It is also similar to the peptide concentration (IC50 ~ 100 nM) which depresses myocardial inotropism in perfused hearts (Angelone et al. 2008), and appears slightly lower than the IC50 value for CST-induced inhibition of the nicotinic cholinergic receptor-mediated catecholamine release in bovine adrenal chromaffin cells (Mahata et al. 1997).
Contracture limitation has been suggested as a very good indicator of I/R injury (Penna et al. 2009c; Gelpi et al. 2002). In fact, both ischemic PostC and CST-Post markedly limited contracture development during reperfusion. Furthermore, CST was somewhat more protective than ischemic PostC, as the improvement of systolic function with CST-Post was greater than that observed with ischemic PostC. Since heart rate and ventricular volume were kept constant, a role for both force–frequency relationship and a Starling effect can be excluded. Therefore, the improved systolic function is suggestive of a direct anti-stunning effect by CST. Whether or not ischemic PostC improves stunning is controversial (Couvreur et al. 2006; Penna et al. 2009a, c). Many authors suggested that the PostC maneuvers (intermittent flow interruption) do not abolish the stunning and that the improvement of global cardiac function, if any, should be due to anti-necrotic effect (Couvreur et al. 2006; Penna et al. 2009a, c). Moreover, increased cell viability does not necessarily correspond to improved systolic function because cell can be viable but stunned. Despite similar anti-infarcting effect by ischemic PostC and CST-Post, we observed a better recovery of systolic function with CST-Post. Accordingly, it is likely that the limitation of post-ischemic contracture is reflected in a CST-elicited increase in dP/dt and anti-stunning effect. Limited contracture is likely due to a reduced calcium overload resulting either from calcium extrusion and/or from increased re-uptake by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). While the former may reduce contractility, the latter tends to increase it. Of note, Angelone et al. (2008) reported that, in the absence of ischemia, CST negatively influences the inotropism. However, our post-ischemic testing of CST in this study and our previous study in normal heart (Angelone et al. 2008) are not directly comparable. Being the aim of this study not mechanistically oriented, the CST anti-contracture and anti-stunning effects deserve further investigation.
It has been shown that several peptides such as bradykinin, opioids, and tumor necrosis factor (TNFα) are able to induce post-conditioning-like cardioprotective effects (Bell and Yellon 2003; Penna et al. 2008a, b; Lecour 2009). These peptides, acting on their specific receptors, can trigger both pharmacological pre- and post-conditioning via pro-survival intrinsic signaling cascades, which include in rodents the so called Reperfusion Injury Salvage Kinases (RISK) and Survivor Activating Factor Enhancement (SAFE) pathways (Heusch et al. 2008; Penna et al. 2008a, b; Lecour 2009). Here, we show that like these peptides, CST is also able to induce cardioprotection either if given during the early reperfusion phase in isolated hearts, or if added during a challenging ischemia in isolated cells. In ongoing experiments, CST, given as a pre-conditioning agent, reduced infarct size and post-ischemic contracture less than CST-Post. Moreover, only CST-Post significantly improved post-ischemic recovery of developed LVP. Therefore, CST seems more protective as PostC agent than as a pre-conditioning mimetic.
Of note, in patients with CHF circulating CgA is increased and is an independent predictive factor for mortality (Ceconi et al. 2002). In particular, CgA correlates with soluble TNF receptors (sTNF-Rs) (Corti et al. 2000). The good correlation between CgA and sTNF-Rs and the lack of correlation with neuroendocrine variables (Corti et al. 2000), suggest that circulating CgA reflects systemic inflammation much better than neuroendocrine activation in CHF (Corti et al. 2000; Ceconi et al. 2002).
Apart from its interaction with nicotinic receptors (Mahata et al. 1997, 2000, 2010), the mechanisms underlying the action of CST at the cardiac level remains to be clarified (Helle et al. 2007, 2010). It has been proposed that CST may interact with the alpha subunit of Gi protein (Helle et al. 2007; Helle 2010). Nevertheless, CST and its analogue VS are able to activate a cascade similar to that involved in cardioprotection when given before ischemia (Cappello et al. 2007; Angelone et al. 2008). In particular, it has been reported that VS cross-reacts with adenosine receptors to induce protection (Cappello et al. 2007).
Since CST activates some elements of the RISK pathway, including nitric oxide synthase, and may antagonize adrenergic effects (Herrero et al. 2002; Angelone et al. 2008), we wondered whether CST may be cardioprotective independently from the endothelial and anti-adrenergic effects. In fact, it is well known that NO plays a cardioprotective role (Penna et al. 2006; Cappello et al. 2007; Heusch et al. 2008) and it has been demonstrated that endothelium-derived NO mediates the VS-1-induced antiadrenergic effect in rat ventricular myocardium (Gallo et al. 2007; Cerra et al. 2008). Moreover, it has been suggested that β1-adrenoreceptor stimulation may be detrimental in the reperfusion phase, thus increasing infarct size (Feuerstein et al. 1998; Gao et al. 2000). Noteworthy, also in human CHF, chronic heightened activation of the sympathetic system and associated enhancement of catecholamine-induced signaling pathways have adverse prognostic significance and may accelerate the pathological processes (Esler et al. 1997). In the heart, catecholamines are co-stored and co-released with other neuropeptides and humoral principles, in the heterogeneous population of afferent, efferent, and interconnecting short neurons and intracardiac ganglia, in the chromaffin cells, the endocardial endothelium, the coronary vessels, and the connective cells of the interstitium, as well as in the myocardiocytes themselves. The latter include the population of intrinsic cardiac adrenergic cells identified in 1996 by Huang et al. (1996) in rodent and in human hearts. Accordingly, these intracardiac converging adrenergic stimuli may significantly augment the adrenergic activation of the heart under stressful conditions. Therefore, since in the isolated rat heart excitatory adrenergic cascades are likely to occur (Chahine et al. 1994), we argue that the observed cardioprotective effects are, at least in part, related to the anti-adrenergic effect of CST (Mahata et al. 1997; Herrero et al. 2002; Angelone et al. 2008). However, since we observed a well evident limitation of I/R injury also in the isolated cardiomyocytes, we suggest that CST is able to attain such protection also via a direct effect on cardiomyocytes, which is independent from catecholamine presence in the extracellular milieu. Furthermore, in reperfusion the protective effect is not obligatorily endothelial-dependent. Our results, however, do not rule out an additional role for the anti-adrenergic and/or endothelium-dependent mechanisms in the in situ heart. In fact, endothelium was required in the negative inotropic effect of vasostatin (Gallo et al. 2007; Cerra et al. 2008). Yet, a higher CST concentration was required in the heart (75 nM) with respect to cardiomyocytes (5 nM), possibly because of hampered mass transfer of the peptide to myocardial target trough the endothelium. Our study does not allow to compare CST potency between isolated cardiomyocytes and whole organ. Likely, on the isolated heart, higher CST concentrations should be used for reaching interstitial peptide levels comparable to the isolated cardiomyocyte experimental conditions.
In conclusion, CST applied in the reperfusion is protective especially in terms of improvement of post-ischemic cardiac function. Since protection is observed in both isolated heart and isolated cardiomyocytes, we suggest that the protective effect is primarily due to a direct effect on the myocardium and does not necessarily depend on the antiadrenergic and/or endothelial effects of CST.
Conceivably, CST influence may be multifunctional, being achieved not only via the baroceptor and sympathoadrenal systems, but also via direct protective mechanisms on cardiomyocytes. Our study may provide insights into the importance of the stimulus-secretion coupling of CgA and its spatio-temporal processing as an attempt of the cardiovascular system to protect itself against I/R damages and associated patho-physiological disturbances.
References
Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC (2008) The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793
Baker JE, Konorev EA, Gross GJ, Chilian WM, Jacob HJ (2000) Resistance to myocardial ischemia in five rat strains: is there a genetic component of cardioprotection? Am J Physiol Heart Circ Physiol 278:H1395–H1400
Bell RM, Yellon DM (2003) Bradykinin limits infarction when administered as an adjunct to reperfusion in mouse heart: the role of PI3K, Akt and eNOS. J Mol Cell Cardiol 35:185–193
Cappello S, Angelone T, Tota B, Pagliaro P, Penna C, Rastaldo R, Corti A, Losano G, Cerra MC (2007) Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signaling mechanism. Am J Physiol Heart Circ Physiol 293:H719–H727
Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A (2002) Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 23:967–974
Cerra MC, De Iuri L, Angelone T, Corti A, Tota B (2006) Recombinant N-terminal fragments of chromogranin-A modulate cardiac function of the Langendorff-perfused rat heart. Basic Res Cardiol 101:43–52
Cerra MC, Gallo MP, Angelone T, Quintieri AM, Pulerà E, Filice E, Guérold B, Shooshtarizadeh P, Levi R, Ramella R, Brero A, Boero O, Metz-Boutigue MH, Tota B, Alloatti G (2008) The homologous rat chromogranin A1-64 (rCgA1-64) modulates myocardial and coronary function in rat heart to counteract adrenergic stimulation indirectly via endothelium-derived nitric oxide. FASEB J 22:3992–4004
Chahine R, Nadeau R, Lamontagne D, Yamaguchi N, de Champlain J (1994) Norepinephrine and dihydroxyphenylglycol effluxes from sympathetic nerve endings during hypoxia and reoxygenation in the isolated rat heart. Can J Physiol Pharmacol 72:595–601
Corti A, Ferrari R, Ceconi C (2000) Chromogranin A and tumor necrosis factor-α (TNF) in chronic heart failure. Adv Exp Med Biol 482:351–359
Couvreur N, Lucats L, Tissier R, Bize A, Berdeaux A, Ghaleh B (2006) Differential effects of postconditioning on myocardial stunning and infarction: a study in conscious dogs and anesthetized rabbits. Am J Physiol Heart Circ Physiol 291:H1345–H1350
Dev NB, Gayen JR, O’Connor DT, Mahata SK (2010) Chromogranin A and the autonomic system: decomposition of heart rate variability and rescue by its catestatin fragment. Endocrinology 151:2760–2768
Esler M, Kaye D, Lambert G, Esler D, Jennings G (1997) Adrenergic nervous system in heart failure. Am J Cardiol 80:7L–14L
Feuerstein G, Liu GL, Yue TL, Cheng HY, Hieble JP, Arch JR, Ruffolo RR Jr, Ma XL (1998) Comparison of metoprolol and carvedilol pharmacology and cardioprotection in rabbit ischemia and reperfusion model. Eur J Pharmacol 351:341–350
Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, Rao F, Stridsberg M, Ziegler M, Mahata SK, O’Connor DT (2010) Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin Exp Hypertens 32:278–287
Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, Alloatti G (2007) Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol 292:H2906–H2912
Gao F, Chen J, Lopez BL, Christopher TA, Gu J, Lysko P, Ruffolo RR Jr, Ohlstein EH, Ma XL, Yue TL (2000) Comparison of bisoprolol and carvedilol cardioprotection in a rabbit ischemia and reperfusion model. Eur J Pharmacol 406:109–116
Gayen JR, Gu Y, O’Connor DT, Mahata SK (2009) Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin A null mouse. Endocrinology 150:5027–5035
Gelpi RJ, Morales C, Cohen MV, Downey JM (2002) Xanthine oxidase contributes to preconditioning’s preservation of left ventricular developed pressure in isolated rat heart: developed pressure may not be an appropriate end-point for studies of preconditioning. Basic Res Cardiol 97:40–46
Hausenloy DJ, Ong SB, Yellon DM (2009) The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol 104:189–202
Helle KB (2010) The chromogranin A-derived peptides vasostatin-I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res 85:9–16
Helle KB, Corti A, Metz-Boutigue MH, Tota B (2007) The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 64:2863–2886
Herrero CJ, Alés E, Pintado AJ, López MG, García-Palomero E, Mahata SK, O’Connor DT, García AG, Montiel C (2002) Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J Neurosci 22:377–388
Heusch G, Boengler K, Schulz R (2008) Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118:1915–1919
Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, Kelly RA, Smith TW (1996) An intrinsic adrenergic system in mammalian heart. J Clin Invest 98:1298–1303
Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K (2009) Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J 30:25–32
Kennedy BP, Mahata SK, O’Connor DT, Ziegler MG (1998) Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides 19:1241–1248
Lecour S (2009) Activation of the protective Survivor Activating Factor Enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol 47:32–40
Mahapatra NR, O’Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, Staite E, Wu H, Gu Y, Dalton N, Kennedy BP, Ziegler MG, Ross J, Mahata SK (2005) Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest 115:1942–1952
Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ (1997) Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100:1623–1633
Mahata SK, Mahata M, Wakade AR, O’Connor DT (2000) Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A(344–364)): identification of amino acid residues crucial for activity. Mol Endocrinol 14:1525–1535
Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O’Connor DT (2004) The catecholamine release-inhibitory “catestatin” fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol 66:1180–1191
Mahata SK, Mahata M, Fung MM, O’Connor DT (2010) Catestatin: a multifunctional peptide from chromogranin A. Regul Pept 162:33–43
O’Connor DT, Deftos LJ (1986) Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med 314:1145–1151
O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ (2002) Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345
O’Connor DT, Zhu G, Rao F, Taupenot L, Fung MM, Das M, Mahata SK, Mahata M, Wang L, Zhang K, Greenwood TA, Shih PB, Cockburn MG, Ziegler MG, Stridsberg M, Martin NG, Whitfield JB (2008) Heritability and genome-wide linkage in US and Australian twins identifies novel genomic regions controlling the catecholamine release-inhibitory peptide catestatin. Circulation 118:247–257
Omland T, Dickstein K, Syversen U (2003) Association between plasma chromogranin A concentration and long-term mortality after MI. Am J Med 114:25–30
Pagliaro P, Mancardi D, Rastaldo R, Penna C, Gattullo D, Miranda KM, Feelisch M, Wink DA, Kass DA, Paolocci N (2003) Nitroxyl affords thiol-sensitive myocardial protective effects akin to early preconditioning. Free Radic Biol Med 34:33–43
Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, Losano G, Pagliaro P (2006) Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol 101:168–179
Penna C, Mancardi D, Raimondo S, Geuna S, Pagliaro P (2008a) The paradigm of postconditioning to protect the heart. J Cell Mol Med 12:435–458
Penna C, Mancardi D, Tullio F, Pagliaro P (2008b) Postconditioning and intermittent bradykinin induced cardioprotection require cyclooxygenase activation and prostacyclin release during reperfusion. Basic Res Cardiol 103:368–377
Penna C, Mognetti B, Tullio F, Gattullo D, Mancardi D, Pagliaro P, Alloatti G (2008c) The platelet activating factor triggers preconditioning-like cardioprotective effect via mitochondrial K-ATP channels and redox-sensible signaling. J Physiol Pharmacol 59:47–54
Penna C, Mancardi D, Tullio F, Pagliaro P (2009a) Intermittent adenosine at the beginning of reperfusion does not trigger cardioprotection. J Surg Res 153:231–238
Penna C, Perrelli MG, Raimondo S, Tullio F, Merlino A, Moro F, Geuna S, Mancardi D, Pagliaro P (2009b) Postconditioning induces an anti-apoptotic effect and preserves mitochondrial integrity in isolated rat hearts. Biochim Biophys Acta 1787:794–801
Penna C, Tullio F, Merlino A, Moro F, Raimondo S, Rastaldo R, Perrelli MG, Mancardi D, Pagliaro P (2009c) Postconditioning cardioprotection against infarct size and post-ischemic systolic dysfunction is influenced by gender. Basic Res Cardiol 104:390–402
Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A (2007) Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 28:1117–1127
Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, Mahata M, Kennedy BP, Salem RM, Stridsberg M, Abel K, Smith DW, Eskin E, Schork NJ, Hamilton BA, Ziegler MG, Mahata SK, O’Connor DT (2007) Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation 115:2271–2281
Rosjo H, Masson S, Latini R, Flyvbjerg A, Milani V, La Rovere M, Revera M, Mezzani A, Tognoni G, Tavazzi L, Omland T (2010) Prognostic value of chromogranin A in chronic heart failure: data from the GISSI-Heart Failure Trial. Eur J Heart Fail 12:549–556
Acknowledgments
The authors were supported by Compagnia di S. Paolo, National Institutes of Cardiovascular Research (INRC, BT, MCC, GA, PP, CP); Regione Piemonte (GA, PP, CP), ex-60% (CP, PP). The authors wish to thank Prof. Donatella Gattullo for insightful suggestions. S.K.M. was supported by grants from the Veterans Affairs and the National Institutes of Health.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Additional information
A commentary to this article can be found at doi:10.1007/s10571-010-9552-6.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Penna, C., Alloatti, G., Gallo, M.P. et al. Catestatin Improves Post-Ischemic Left Ventricular Function and Decreases Ischemia/Reperfusion Injury in Heart. Cell Mol Neurobiol 30, 1171–1179 (2010). https://doi.org/10.1007/s10571-010-9598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-010-9598-5