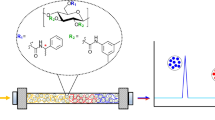
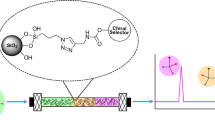
Abstract
Novel chiral selectors based on cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(α-phenylethyl carbamate) were regioselectively synthesized by carbonate aminolysis and isocyanate chemistry. By oxycarbonylation with phenyl chloroformate, carbamoylation with 3,5-dimethylphenyl isocyanate, and subsequent aminolysis of the previously introduced reactive carbonate moiety at C6 with enantiopure (R)-or (S)-α-phenylethylamine, chiral selectors have been obtained, which regioselectively carry two different phenyl carbamate substituents. The cellulose derivatives were comprehensively characterized by ATR-FTIR, solid-state NMR, GPC, and elemental analysis. In parallel, 3-aminopropyl-functionalized silica gel as an inert carrier material for the chiral selectors was prepared and the obtained coated-type chiral stationary phases were characterized by both solid-state 29Si NMR, 13C NMR, and elemental analysis. The enantioseparation performance of the chiral selectors was studied and compared to cellulose tris(3,5-dimethylphenyl carbamate) as a reference. With this protocol in hand, certain shortcomings of conventional approaches towards the regioselective synthesis of polysaccharide-based chiral selectors were overcome, such as the limitation to standard isocyanate reagents, being able to apply now the whole wealth of commercially available (chiral) primary and also secondary alkylamines instead.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemistry of living matter is well defined by asymmetric characteristics of naturally occurring organic compounds, i.e., the presence of stereoisomers/enantiomers which exhibit different properties in chiral biological/biochemical systems (Pasteur 1858; Berthod 2006). In 1848, Louis Pasteur mechanically separated tartrate enantiomers under a microscope, which eventually led to the development of the concept of chirality (Pasteur 1858; D’Orazio 2020). However, the separation of enantiomers is rarely possible by mechanical means. Nowadays, mostly chiral stationary phases in high-performance liquid chromatography (HPLC) are used for the direct separation of pairs of enantiomers and other stereoisomers – both on an analytical and a preparative scale (Ahuja 1997; Subramanian 2008). Another separation approach is the use of chiral (enantiopure) mobile phase additives for the formation of transient diastereomeric species in HPLC – besides other indirect approaches, such as derivatization with chiral species and subsequent separation of the resulting diastereomers (Peng et al. 2016; Yu et al. 2019; Ao et al. 2022; Chen et al. 2022; Bao et al. 2022). Chiral/asymmetric synthesis (Xue et al. 2018; Wojaczyńska and Wojaczyński 2020; Qian et al. 2021; Zhu et al. 2021; Sun et al. 2022) and the kinetic resolution of enantiomers (Musa 2020; Spelmezan et al. 2020; Harwood et al. 2021; Huang et al. 2022; Yang et al. 2022), e.g., by enzymes, as approaches to obtain enantiopure compounds or at least non-racemic mixtures are to be mentioned as well.
The trailblazer for the direct enantioseparation with chiral selectors (CSs) based on cellulose derivatives was inter alia the work by Hesse and Hagel (1973) who applied cellulose triacetate as a CS in chromatography (Hesse and Hagel 1973). Since then, the importance of enantioseparation has been constantly increasing: in stereoselective synthesis (D’Orazio 2020), the analysis of enantiomers in food products (Rocco et al. 2013; Fanali et al. 2019; Alvarez-Rivera et al. 2020), insecticides (Zhao et al. 2019), pesticides (Carrão et al. 2019), herbicides (Martín-Biosca et al. 2001; Lao and Gan 2006; Jin et al. 2010), fungicides (Ying et al. 2009), and in pharmaceutical products (Mukherjee and Bera 2012; Singh et al. 2020) due to the different properties of enantiomers in biological systems (Tang et al. 2011; Yin et al. 2019a).
A series of homo-substituted cellulose and amylose derivatives carrying phenyl carbamate and/or benzoate moieties has been mainly developed by the Y. Okamoto, B. Chankvetadze, and E. Francotte research groups, and the respective CSPs were shown to possess a very high chiral discrimination capacity (Okamoto et al. 1986; Okamoto and Kaida 1994; Minguillón et al. 1996; Tang et al. 2010; Fanali et al. 2019) and to separate up to 90% of all tested racemic compounds or enantiomeric mixtures (Ikai and Okamoto 2009; Shen and Okamoto 2016; Yin et al. 2019a).
In some cases, hetero-substituted cellulose derivatives were shown to have an even higher chiral separation performance in comparison to the respective homo-substituted derivatives (Acemoglu et al. 1998; Felix 2001; Katoh et al. 2011; Yin et al. 2019b). In 1993, Kaida and Okamoto pioneered the regioselective synthesis of hetero-substituted cellulose-type CSs, i.e., derivatives with more than one type of substituents, using sequential tritylation of C6–OH in cellulose and amylose followed by carbamoylation at C2–OH and C3–OH with isocyanates and subsequent deprotection (detritylation) (Kaida and Okamoto 1993). Various CSs based on cellulose derivatives with different phenyl carbamate and/or benzoate substituents have been reported since then (e.g., (Chassaing et al. 1996; Zheng et al. 2009; Shen et al. 2018). Several studies highlighted that the substituent at C6 of the AGU of the polysaccharide backbone had a significant influence on chiral recognition (Kaida and Okamoto 1993; Chassaing et al. 1996; Acemoglu et al. 1998; Felix 2001). The synthesis pathways towards these hetero-substituted cellulose CSs are typically rather laborious, chemical- and time-consuming, and they involve protecting group chemistry in combination with reactive isocyanate reagents. Chassaing et al. 1997 reported that in the case of hetero-substituted celluloses a chiral phenyl carbamate at C6 besides achiral phenyl carbamates at C2 and C3 had better enantioseparation than the counterpart homo-substituted with phenyl carbamates (Chassaing et al. 1997).
Besides the chemical structure of the CS, the enantioseparation performance of a coated chiral stationary phase is significantly dependent on the polysaccharide raw material, its molecular weight (Ichida et al. 1984; Chassaing et al. 1997; Okada et al. 2016; Zhang et al. 2020), the coating amount, the coating procedure and solvent used (Yashima et al. 1996; Wei et al. 2019), as well as silica gel characteristics, such as particle size, dispersity, or pore size (Yashima et al. 1996; Qin et al. 2010; Bezhitashvili et al. 2017; Kohout et al. 2019), and of course the respective analytes, the mobile phase and HPLC conditions (Yashima et al. 1996; Bui et al. 2021). To ensure an objective comparison and proper evaluation of the enantioseparation performance of novel CSs, all parameters except the actual chemical structures of these CSs should of course be kept constant.
Cellulose carbonates are a convenient platform for synthesizing cellulose derivatives, which is especially true for cellulose carbamate synthesis via aminolysis of the carbonates with simple primary and secondary alkyl amines (Pourjavadi et al. 2011; Elschner et al. 2013; Ganske and Heinze 2018). Cellulose aryl carbonates were shown to be more reactive than alkyl carbonates (Elschner and Heinze 2015). For details on carbonate aminolysis to obtain cellulose carbamates see the comprehensive studies by T. Heinze and others (Pourjavadi et al. 2011; Elschner et al. 2013; Elschner and Heinze 2015; Ganske and Heinze 2018). Different phenyl chloroformates have inter alia been evaluated as reagents for cellulose aryl carbonate synthesis and it was reported that most phenyl chloroformates react regioselectively (but not regiospecifically) with C6–OH, rather than with C2–OH and C3–OH groups of cellulose (Elschner et al. 2014; Ganske and Heinze 2018).
To the best of our knowledge, a compatible combination of isocyanate chemistry and oxycarbonylation/aminolysis has not yet been reported for the synthesis of hetero-substituted cellulose CSs. In this study, we present a sequence consisting of regioselective oxycarbonylation reaction with phenyl chloroformate at C6–OH, subsequent quantitative carbamoylation of C2–OH and C3–OH by isocyanates, and aminolysis of the reactive carbonate moieties, for the controlled synthesis of CSs bearing different substituents. The reactive carbonate moieties in this case are acting both as protecting group and reactive moiety for carbamate synthesis via aminolysis. The synthesis conditions for all steps were comprehensively optimized. The chemical structures of the cellulose derivatives, intermediates, and the target CSs were analyzed by FTIR, solid-state 13C CP/MAS NMR, GPC, and elemental analysis (EA). In parallel, 3-aminopropyl-functionalized silica gel as a carrier for the CSs was prepared from silica and trialkoxysilane precursors and characterized by both solid-state 29Si CP/MAS and 13C CP/MAS NMR as well as EA.
The enantioseparation performance of the CSs was evaluated by HPLC in comparison to two references, an in-house prepared CS and a commercially available chiral column, both being based on the common cellulose tris(3,5-dimethylphenyl carbamate) CS.
Results and discussion
Synthesis of the reference compound for structural comparison
Cellulose tris(phenyl carbonate) 1 (Fig. 1A) was synthesized as a reference compound for structural comparison to cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(phenyl carbonate) 3, the latter being the precursor for the synthesis of the novel CSs 4R and 4S. The reaction has been optimized towards an almost quantitative conversion of all hydroxy groups of the polysaccharide backbone.
A Chemical synthesis of the reference compound cellulose tris(phenyl carbonate) 1; B synthesis of the reference chiral selector cellulose tris(3,5-dimethylphenyl carbamate) 2; and C synthesis of the novel chiral selectors cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(α-phenylethyl carbamate) 4R and 4S. Conditions: a DMAc/LiCl/pyridine, 0 °C, 12 h, N2; b pyridine, 110 °C, 24 h, N2; c DMAc/LiCl/pyridine, 0 °C, 12 h, N2; d DMAc/LiCl/pyridine, 80 °C, 18 h, N2; e DMF, 50 °C, 24 h, N2
The oxycarbonylation reaction was evaluated by ATR-FTIR and the chemical structure of cellulose derivative 1 was confirmed by solid-state 13C NMR. Exemplary spectra are presented in Fig. S1 and Fig. S2, respectively (see Supplementary Information). The absence of IR bands from free OH groups at 3400 cm−1, the presence of bands assigned to C=O at 1763 cm−1, C=C aromatic rings at 1592, 1492, 1457, 835 cm−1, as well as C-O at 1236 and 1205 cm−1 indicate that microcrystalline cellulose, the starting material used, was quantitatively oxycarbonylated. The FTIR spectrum of cellulose derivative 1 was fully consistent with literature data (Ganske and Heinze 2018). The solid-state 13C CP/MAS NMR spectrum with carbon signals assigned to the C=O group at 152.9 ppm and the aromatic ring at 120.7, 126.1, 129.1, and 150.9 ppm confirmed this finding (Fig. 2). The signal of the oxycarbonylated C6 was shifted downfield to 66.3 ppm.
Synthesis of the reference chiral selector
Cellulose tris(3,5-dimethylphenyl carbamate) was synthesized as a reference CS (Fig. 1B). This type of CS is a standard material in chiral separation columns and has been commercialized by several companies, e.g., Daicel Corp. and Phenomenex Inc. However, to compensate for differences in silica dimensions, cellulose starting material, column dimensions, coating procedure, and packing, we have decided to synthesize the reference CS in-house, allowing for a fairer comparison between the reference CS and novel synthesized CSs (4R and 4S) since in this case all materials were processed in the same way. FTIR and solid-state 13C NMR spectra of cellulose derivative 2 are shown in Fig. S3 and Fig. S4, and the related EA results are shown in Table 1, which also gives the values for the other cellulose derivatives synthesized. The FTIR spectrum of cellulose derivative 2 shows bands related to N-H at 3320, C=O at 1722, aromatic C=C at 1615, 1539, 1455, 839, and C-O at 1213 cm−1, and the absence of bands from free OH groups in cellulose around 3400 cm−1, in agreement with literature data (Liu et al. 2013; Wei et al. 2019). The solid-state 13C NMR showed resonances at 152.6 (C=O), 138.3, 125.0, 116.1 (aromatic ring), and 20.8 ppm (CH3), confirming successful carbamoylation (Fig. S4). The chemical shift in solid-state 13C NMR of the carbamoylated C6 was 64.9 ppm. The DS of the 3,5-dimethylphenyl carbamate moiety calculated based on the N content (EA) was 2.93 (98%). The molecular weight and dispersity of cellulose derivative 2 were 172.3 kDa (Mw) and 1.70 (Đ), respectively.
Synthesis of the precursor of the selectors
Oxycarbonylation with phenyl chloroformate at C6–OH, followed by carbamoylation with isocyanates at C2–OH and C3–OH, as a one-pot procedure, was used for the synthesis of compound 3 (Fig. 1C). Phenyl chloroformate was chosen due to higher reactivity in this case in comparison to p-nitrophenyl and 4-chlorophenyl chloroformates. The FTIR spectra of cellulose derivative 3 are shown in Fig. S5. The chemical structure of cellulose derivative 3 was additionally confirmed by solid-state 13C NMR analysis in comparison to derivatives 1 and 2. The respective spectra are shown in Fig. 2.
The absence of free OH groups (no bands around 3400 cm−1), and bands for N-H at 3370 cm−1, C=O at 1742 cm−1 (superposition of carbonate and carbamate carbonyls), C=C aromatic ring signals at 1612, 1543, 1453, 839 cm−1, and C-O at 1207 cm−1 in the FTIR spectrum indicated that both oxycarbonylation and carbamoylation reactions proceeded successfully. The carbonyl signals of cellulose derivative 3 were at 1742 cm−1 in comparison to cellulose derivative 1 (1763 cm−1) and cellulose derivative 2 (1722 cm−1). The carbonate substituent, introduced first, remained stable during the subsequent carbamoylation reaction at elevated temperature (80 °C), which was also confirmed by solid-state 13C NMR: the “marker” signals of C9 and C10 at 121.0 and 129.1 ppm, respectively, indicate the phenyl carbonate moieties on cellulose derivative 3, while the signals of C13 and C15 at 138.0, C14 at 115.8 and C17 at 20.8 ppm confirm the presence of the 3,5-dimethylphenyl carbamate substituents. The C6 resonance at 67.0 ppm indicates that C6 is solely oxycarbonylated and not carbamoylated (see the downfield shift in comparison to compound 2 (64.9 ppm) in Fig. 2). The DS of the 3,5-dimethylphenyl carbamate based on the N content (EA) was calculated to be 2.05; the DS of phenyl carbonate was 0.93 (see Supplementary Information).
Synthesis of the novel chiral selectors by carbonate aminolysis
Oxycarbonylation at C6–OH, while having C2–OH and C3–OH carbamoylated, provides a “reactive protecting group” to be further modified by aminolysis. This converts the carbonate moiety into a carbamate. In this way, compound 3 was brought to reaction with two enantiopure alkyl amines: (R)- and (S)-α-phenylethyl amine. Virtually any primary or even secondary alkyl amine can be used in this way for obtaining carbamate structures, and the scope thus goes far beyond the relatively few functionalities that are accessible today by standard isocyanate reagents. It is important to point out that aromatic amines cannot be used for aminolysis of the carbonates due to their low nucleophilicity, which was already shown in a comprehensive study by (Elschner et al. 2013).
The FTIR spectra of cellulose derivatives 4R and 4S vs. 3 are shown in Fig. S6 and Fig. S7, respectively. The change of the carbonyl band from 1742 to approx. 1710 cm−1 (carbamate) and the significant decrease in the intensity of C-O signals at 1217 cm−1 indicate that the aminolysis reaction was successful. The solid-state 13C NMR spectra of cellulose derivatives 4R and 4S vs. 3 are shown in Fig. 3 and Fig. S8. Characteristic resonances appear for C18 at approx. 51 ppm, C20 at 143 ppm, and C22 at 127 ppm. The chemical shift of C7 changes from 151.7 in the carbonate to 154 ppm in the carbamate substituent, as well as the shift of the C6 from 67 ppm in the carbonate to 63 ppm in the product. The absence of resonances of the phenyl carbonate moiety (C9 and C10, Fig. 3 and Fig. S8) confirmed that the aminolysis reactions proceeded nearly quantitatively. The degrees of substitution of the R- or S-α-phenylethyl carbamates, based on the N content from elemental analysis, were 0.78 and 0.75, respectively. The molecular weights and dispersities of the cellulose derivatives 4R and 4S were 238.8 and 234.2 kDa (Mw), and 3.53 and 2.76 (Ɖ), respectively.
Pre-functionalization of silica gel
The different silicon species and their resonances in solid-state 29Si NMR adapted from (Salon et al. 2007) are presented in Fig. 4A. The solid-state 29Si CP/MAS NMR spectra of APS vs. the non-modified silica gel starting material and the respective solid-state 13C NMR spectrum of APS are shown in Fig. 4B C. The EA results for APS are shown in Table 1. The T2 and T3 resonances at approx. −60 and −70 ppm, respectively, in the solid-state 29Si NMR spectrum of APS indicate successful covalent modification of the silica gel, as does the presence of 13C resonances (solid-state NMR) (Cγ at approx. 42, Cβ at approx. 22, and Cα at approx. 10 ppm). The loading of aminopropyl groups, calculated based on the N content from elemental analysis, was 170 µmol/g (0.96 wt% N).
Evaluation of the enantioseparation performance
A series of ten chiral analytes (α-methyl-D,L-phenylalanine methyl ester a, Tröger’s base b, a mixture of pseudoenantiomeric tert-butylcarbamoyl quinine and tert-butylcarbamoyl quinidine (ratio of 2:1, c), 2-phenylcyclohexanone d, benzoin e, Pirkle’s alcohol f, trans-stilbene oxide g, mandelic acid isopropyl and methyl ester derivatives h and i, and 1-(o-hydroxyphenyl)-3-phenyl-1-propanol j, see Fig. 5) was used to study the enantioseparation performance of the developed column materials (CSP4R and CSP4S) in comparison to an in-house reference column (CSP2) and a commercial reference column (CHIRALCEL® OD).
The chromatographic retention factor (k1), selectivity (α), and resolution (Rs) data of the chiral analytes for all tested CSPs are summarized in Fig. 6 and Table S1. Example HPLC chromatograms for the chiral analytes b and j are shown in Fig. 7.
All of the selected chiral analytes could be – at least partially – separated on both the in-house prepared reference material CSP2 and the commercially available CHIRALCEL® OD column. Especially, the chiral analytes e, f, g, and i with Rs values > 1.5 were baseline-separated, while the chiral analytes a, b, and d with 0 < Rs < 1.5 were at least partly separated. The analytes c and j were partly separated on CHIRALCEL® OD with Rs = 0.17 and 0.58, respectively, but not separated on CSP2. Chiral analyte h was baseline separated on CHIRALCEL® OD with Rs = 1.86, but only partly on CSP2 (Rs = 1.27). Analytes a, b, d, f, and i were better resolved on CSP2, analytes e and g on CHIRALCEL® OD. The highest selectivity and resolution among the tested chiral analytes on both CSP2 and CHIRALCEL® OD was seen for analyte f (α = 3.19 and 2.97, Rs = 4.76 and 4.60, respectively). Overall, the resolution values in the case of the reference CSP2 were quite comparable to the commercial column CHIRALCEL® OD. The CHIRALCEL® OD column was added to this study as a commercially available benchmark column. Note some differences in silica gel dimensions (10 vs. 7 μm) and column dimensions (250 × 4.6 vs. 150 × 4 mm) for CHIRALCEL® OD and CSP2, respectively.
Regarding the enantioseparation performance of CSP4R and CSP4S, the chiral analytes a, b, d, e, f, g, and j were partly separated on these columns with Rs values < 1.5. The analytes b, d, e, and f were better separated on CSP4S, while analytes a and j had higher resolution on CSP4R. The analytes c, h, and i were partly separated on CSP4R (Rs = 0.47, 0.37, and 0.61, respectively), but not on CSP4S. The chiral analytes b and d gave higher resolution values on CSP4R/CSP4S than on the reference column CSP2, while CSP2 showed higher enantioseparation for the chiral analytes a, e, f, and g (see Fig. 6 and Table S1). The chiral analytes h and i gave higher resolution values on CSP2 compared to CSP4R, while they could not be separated on CSP4S with the applied mobile phase conditions. The analyte j was partly separated on both CSP4R and CSP4S with Rs = 0.94 and 0.36, respectively, however, it could not be separated on CSP2. Analyte c was partly separated on CSP4R (Rs = 0.47), but it could not be separated on both CSP4S and CSP2. It is noteworthy that the chiral analytes c and j had a higher resolution on CSP4R with Rs = 0.47 and 0.98 than on CHIRALCEL® OD with Rs = 0.17 and 0.58, and they could not be separated at all on CSP2.
Conclusion
Carbonate aminolysis and isocyanate chemistry were exploited to obtain novel cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(α-phenylethyl carbamate)-based CSs. The regioselective oxycarbonylation of C6–OH of the polysaccharide backbone of cellulose with phenyl chloroformate for obtaining a “reactive protecting group” for later aminolysis of the carbonate moieties was optimized. The hydroxy groups at C2 and C3 of the intermediate were subsequently carbamoylated with 3,5-dimethylphenyl isocyanate in the second step of this one-pot procedure, having a DS of 3,5-dimethylphenyl carbamate of 2.05 and a DS of the phenyl carbonate of 0.93. Aminolysis of the reactive carbonate moiety with two exemplary enantiopure amines – (R)- and (S)-α-phenylethyl amine – yielded novel hetero-substituted cellulose-based CSs. This protocol overcomes the limitations of conventional approaches to CSs, set by the limited variety of isocyanates available. With this protocol in hand, cellulose carbamates are accessible from simple alkyl amines, which offer much wider substrate choices, without the need to resort solely to isocyanate chemistry. Two enantiopure compounds have been used as exemplary amines. However, virtually any primary and also secondary alkyl amine could be used, and this wealth of options will be exploited in our future work to optimize enantioseparation. The obtained chiral selectors were coated onto silica and the obtained CSPs were compared to an in-house prepared reference material and a commercial column material. The newly developed CSPs have shown good overall separation performance, well comparable to the commercial references, although no clear trends can be identified at the moment. Some of the chiral analytes were even better separated on the novel CSPs than with the reference columns. We hope that with this work we have been able to demonstrate that the synthesis of regioselectively modified chiral selectors by oxycarbonylation and subsequent aminolysis is advantageously possible, that the resulting hetero-substituted cellulose derivatives can be used as CSs for enantioseparation in chiral HPLC, and that this approach is certainly worth further exploitation.
Materials and methods
Materials
Microcrystalline cellulose (Avicel® PH-101), p-cymene (99%), α-methyl-D,L-phenylalanine methyl ester (98%, a), Tröger’s base (98%, b), and trans-stilbene oxide (98%, g) were purchased from Sigma-Aldrich (Schnelldorf, Germany). Microcrystalline cellulose was dried at 40 °C in a vacuum oven for at least two days before use. Pseudoenantiomeric tert-butylcarbamoyl quinine and tert-butylcarbamoyl quinidine c were kindly provided by Assoc.Prof. Dr. Michal Kohout (University of Chemistry and Technology, Prague, Czech Republic). 3,5-Dimethylphenyl isocyanate (> 98%), phenyl chloroformate (> 98%), (R)-(+)-α-phenylethyl amine (> 99%), (S)-(-)-α-phenylethyl amine (> 98%), 2-phenylcyclohexanone (> 98%, d), benzoin (> 98%, e) and Pirkle’s alcohol (> 99%, f) were purchased from TCI Europe N.V. (Zwijndrecht, Belgium). The mandelic acid derivatives (h and i) and 1-(o-hydroxyphenyl)-3-phenyl-1-propanol j were synthesized in-house according to standard procedures. Silica gel (7 μm, 1000 Å) was purchased from Daisogel Osaka Soda Co., Ltd. (Japan). Empty stainless HPLC columns (150 × 4 mm, i.d.) and column hardware were purchased from Bischoff Analysentechnik u. -geräte GmbH (Leonberg, Germany). A commercial CHIRALCEL® OD column (10 μm silica gel, 250 × 4.6 mm, i.d.) from Daicel Chiral Technologies Europe SAS (Illkirch Cedex, France) was used for comparative purposes. The organic solvents such as N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), toluene, and pyridine were all reagent grade and dried over 3 Å molecular sieves (Sigma-Aldrich, Schnelldorf, Germany) for at least three days before use. Ethanol (EtOH) and methanol (MeOH) for precipitation and washing were of technical grade and obtained from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) or Fisher Scientific (Vienna, Austria). The HPLC solvents n-hexane (95%) and 2-propanol (99.9%) were obtained from Fisher Scientific.
Instrumentation
ATR-FTIR spectra were recorded on a Frontier IR Single-Range spectrometer (PerkinElmer, Waltham, Massachusetts, US) equipped with a diamond/ZnSe crystal, LiTaO3 detector, and KBr windows. FTIR spectra were evaluated using SpectraGryph software (version v1.2.15). Solid-state 13C CP/MAS (12 kHz) and 29Si CP/MAS (8 kHz) NMR experiments were carried out with an Avance III HD instrument (Bruker, Rheinstetten, Germany) with a resonance frequency of 100.67 MHz for 13C and 79.53 MHz for 29Si. Data processing was carried out with ACD/NMR Processor Academic Edition 12.01 and TopSpin 3.6.2 software (Bruker). Chemical shifts (δ) are given in ppm. Elemental analyses were carried out at the microanalytical laboratory of the University of Vienna with a EURO EA 3000 CHNS-O instrument (HEKAtech, Wegberg, Germany), and halide contents were determined by argentometry. GPC analyses were performed according to standard procedures (Jusner et al. 2022). An Agilent Technologies, Inc. (Santa Clara, CA, USA) 1100 HPLC apparatus equipped with a quaternary pump (G1311A), autosampler (G1313A), and DAD (G1315A) was used to evaluate the enantioseparation performance of the chiral columns. OpenLab CDS software (Agilent) was used for chromatography data processing and evaluation.
Synthesis
Synthesis of cellulose tris(phenyl carbonate) 1
Cellulose tris(phenyl carbonate) was synthesized according to a protocol by (Ganske and Heinze 2018) with modification. Microcrystalline cellulose (1.0 g) was immersed and vigorously stirred in anhydrous DMAc (30 mL) under a dry nitrogen atmosphere. The suspension was heated at 120 °C for 2 h. Anhydrous LiCl (1.8 g) was slowly added after cooling down to < 90 °C, and the mixture was continuously stirred at RT until a clear solution was formed. Anhydrous pyridine (3.0 mL) was slowly added to the solution, which was then cooled to 0 °C by an ice/water bath. Phenyl chloroformate (6 molar equivalents with respect to the AGU repeating unit of cellulose) was added dropwise to the solution and the mixture was allowed to stir for 12 h. The mixture was added to a large excess of distilled water (DW) for the precipitation of crude cellulose derivative 1, which was then collected by vacuum filtration, washed with a large excess of DW and with EtOH (2 × each), and dried at 40 °C in a vacuum oven for two days. Crude cellulose derivative 1 was re-dissolved in acetone (250 mL) and residual LiCl was separated by vacuum filtration through a sintered frit. Acetone was evaporated for the most part by rotary evaporation under reduced pressure and cellulose derivative 1 was precipitated in EtOH, collected by vacuum filtration, washed with a large excess of EtOH and DW (2 × each), and dried at 40 °C in a vacuum oven for two days. Yield: 2.66 g, 83 wt%. Cellulose derivative 1 is soluble in DMAc, acetone, pyridine, DMF, and THF.
Synthesis of cellulose tris(3,5-dimethylphenyl carbamate) 2
Synthesis protocols based on (Okamoto et al. 1984) and (Miaomiao et al. 2017) were applied with modifications to synthesize cellulose tris(3,5-dimethylphenyl carbamate) as a reference CS. Microcrystalline cellulose (2.0 g) was immersed and vigorously stirred in anhydrous pyridine (40 mL) under a dry nitrogen atmosphere. 3,5-Dimethylphenyl isocyanate (6 molar equivalents with respect to the AGU repeating unit of cellulose) was added dropwise. The temperature of the suspension was increased to 110 °C and the carbamoylation reaction was continued for 24 h. A large excess of MeOH was used to precipitate crude cellulose derivative 2, which was then collected by vacuum filtration, washed with MeOH and with DW (2 × each), and then dried in a vacuum oven at 40 °C for two days. Further purification of cellulose derivative 2 was performed by re-dissolution in pyridine and re-precipitation in MeOH, vacuum filtration, and washing of the precipitate with MeOH and with DW (2 × each). The purified cellulose derivative 2 was dried in a vacuum oven at 40 °C for two days. Yield: 5.73 g, 77 wt%. Cellulose derivative 2 is soluble in pyridine, acetone, DMAc, DMF, and THF, but not in acetonitrile. Swelling was observed in CHCl3.
One-pot two-step synthesis of cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(phenyl carbonate) 3
Microcrystalline cellulose (3.0 g) was immersed and vigorously stirred in anhydrous DMAc (90 mL) under a dry nitrogen atmosphere. The mixture was heated at 120 °C for 2 h. After cooling down to < 90 °C, anhydrous LiCl (5.4 g) was slowly added to the suspension and the mixture was continuously stirred at RT until a clear solution was formed. Anhydrous pyridine (9.0 mL) was then added to the solution, which was cooled down to 0 °C by an ice/water bath. Phenyl chloroformate (1.5 molar equivalents with respect to the AGU repeating unit of cellulose) was added dropwise. The oxycarbonylation reaction was carried out for 12 h. Then, anhydrous pyridine (30 mL) was added to the solution, which was heated to 80 °C, followed by the dropwise addition of 3,5-dimethylphenyl isocyanate (5 molar equivalents with respect to the AGU repeating unit of cellulose). The carbamoylation reaction was performed for 18 h at 80 °C. The solution was cooled down to RT and a large excess of DW was used to precipitate crude cellulose derivative 3, which was collected by vacuum filtration, washed with a large excess of DW and with EtOH (2 × each), and dried at 40 °C in a vacuum oven for two days. Crude cellulose derivative 3 was re-dissolved in acetone (750 mL), and residual LiCl was separated by vacuum filtration through a sintered glass frit. Acetone was evaporated for the most part by rotary evaporation under reduced pressure and cellulose derivative 3 was re-precipitated in a large excess of EtOH, collected by vacuum filtration, washed with a large excess of EtOH and with DW (2 × each), and dried at 40 °C in a vacuum oven for two days. Yield: 9.76 g, 92 wt%. Cellulose derivative 3 is soluble in DMAc, pyridine, acetone, DMF, and THF.
Synthesis of cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-((R/S)-α-phenylethyl carbamate) 4 R and 4 S
Cellulose derivative 3 (2.5 g) was stirred in anhydrous DMF (50 mL) under a dry nitrogen atmosphere until a clear solution was formed. Either (R)-(+)-α-methylbenzylamine or (S)-(-)-α-methylbenzylamine (5 molar equivalents with respect to the repeating unit of cellulose derivative 3) was added dropwise, and the aminolysis reactions were performed at 50 °C for 24 h. After cooling down to RT, a large excess of EtOH was used to precipitate crude cellulose derivatives 4R or 4S, which were then collected by vacuum filtration, and washed with a large excess of EtOH and DW (2 × each). Crude cellulose derivative 4R or 4S was re-dissolved in DMF, re-reprecipitated in a large excess of EtOH, collected by vacuum filtration, and washed with a large excess of EtOH and with DW (2 × each). Purified cellulose derivative 4R or 4S was dried at 40 °C in a vacuum oven for 2 days. Yield 4R: 2.37 g, 91 wt%; 4S: 2.42 g, 93 wt%. Cellulose derivatives 4R and 4S are soluble in pyridine, acetone, DMAc, DMF, and THF, but not in acetonitrile. Swelling was observed in CHCl3.
Synthesis of 3-aminopropyl-functionalized silica gel (APS)
The protocols reported by (Engelhardt and Orth 1987; Yashima et al. 1996; Okada et al. 2016) were adapted to synthesize 3-aminopropyl-functionalized silica gel (APS) as an inert carrier for the CSs. Silica gel (10 g) was immersed in toluene (200 mL), mechanically stirred, and the mixture was dried by azeotropic distillation in a dry nitrogen atmosphere. After distilling off about half of the volume of toluene, the temperature of the suspension was reduced to 80 °C, triethylamine (1 mL) was added as a catalyst, followed by the addition of (3-aminopropyl)triethoxysilane (10 mL). The reaction mixture was allowed to stir for 48 h. The suspension was then cooled down to RT and crude APS was collected by vacuum filtration through a sintered glass frit (DURAN®, porosity 4), washed with hot toluene (100 mL), EtOH (100 mL), and with DW (2 × 200 mL). APS was then dried at 40 °C in a vacuum oven for two days. Yield: 9.96 g.
CSP preparation and HPLC method
Silica coating
The coating amount of each CS on APS was 20 wt% (Zhang and Francotte 1995). The coating was performed in three steps for the most homogeneous possible distribution of the CS on APS (Wei et al. 2019). CS (0.4 g) was dissolved in THF (40 mL) and the homogeneous solution was transferred to a round-bottomed flask that contained dry APS (3.2 g). THF was slowly evaporated at 40 °C at 357 mbar in a rotary evaporator. Then, a solution of CS (0.2 g) dissolved in THF (20 mL) was added to the round-bottomed flask, and the solvent was evaporated again. The coating process was repeated once more. After solvent evaporation and drying in a vacuum oven at 40 °C overnight, the coated silica particles were sieved through an analytical sieve before packing (40 μm mesh size).
Column packing
The coated and sieved silica particles (2.3 g) were suspended in a mixture of isopropanol (20 mL) and acetic acid (100 µL). The mixture was sonicated for 20 min to form a homogeneous slurry, which was then packed in-house under high pressure (max. 290 bar) into an empty stainless steel HPLC column. As a compacting agent, MeOH was used (app. 120 mL). After packing, the columns were rinsed with isopropanol until use.
HPLC method
The columns packed with CSP2 (CS = cellulose derivative 2), CSP4R (CS = 4R), and CSP4S (CS = 4S) were rinsed with n-hexane/isopropanol in a stepwise gradient (30:70, 60:40, 90:10, v/v) before use. The concentration of the analytes was 1 mg/mL each. The flow rate and injection volume were set to 1 mL/min and 5 µL, respectively. The absorbance of all analytes was recorded at 254 nm. p-Cymene was used to determine the dead time (t0). All measurements were carried out in triplicate.
References
Acemoglu M, Küsters E, Baumann J, Hernandez I, Pong-Mak C (1998) Synthesis of regioselectively substituted cellulose derivatives and applications in chiral chromatography. Chirality 10(4):294–306
Ahuja S (1997) Chiral Separations and Technology: An Overview. In: Ahuja S (ed) Chiral separations: applications and technology. Amer Chemical Society, Washington DC
Alvarez-Rivera G, Bueno M, Ballesteros-Vivas D, Cifuentes A (2020) Chiral analysis in food science. Trends Analyt Chem 123:115761
Ao Q, Zhao H, Tong T, Peng Y, He Z (2022) Enantioseparation of basic drugs by reverse phase high-performance liquid chromatography system using carboxymethyl‐β‐cyclodextrin as chiral mobile phase additive. Chirality 34(8):1128–1139
Bao H, Sun W, Sun H, Jin Y, Gong X, Chu C, Tong S (2022) Liquid chromatographic study of two structural isomeric pentacyclic triterpenes on reversed-phase stationary phase with hydroxypropyl-β-cyclodextrin as mobile phase additive. J Pharm Biomed Anal 207:114420
Berthod A (2006) Chiral recognition mechanisms. Anal Chem 78 (7):2093–2099
Bezhitashvili L, Bardavelidze A, Ordjonikidze T, Chankvetadze L, Chity M, Farkas T, Chankvetadze B (2017) Effect of pore-size optimization on the performance of polysaccharide-based superficially porous chiral stationary phases for the separation of enantiomers in high-performance liquid chromatography. J Chromatogr A 1482:32–38
Bui CV, Rosenau T, Hettegger H (2021) Polysaccharide-and β-Cyclodextrin-based chiral selectors for enantiomer resolution: Recent developments and applications. Molecules 26(14):4322
Carrão DB, Perovani IS, de Albuquerque NCP, de Oliveira ARM (2019) Enantioseparation of pesticides: A critical review. Trends Analyt Chem 122:115719
Chassaing C, Thienpont A, Félix G (1996) Regioselective carbamoylated and benzoylated cellulose for the separation of enantiomers in high-performance liquid chromatography. J Chromatogr A 738(2):157–167
Chassaing C, Thienpont A, Soulard MH, Felix G (1997) Regioselectivity carbamoylated polysaccharides for the separation of enantiomers in high-performance liquid chromatography. J Chromatogr A 786(1):13–21
Chen B, You H, Fang L, Lin T, Xu P, Chu C, Tong S (2022) Enantioseparation of five racemic N-alkyl drugs by reverse phase HPLC using sulfobutylether‐β‐cyclodextrin as a chiral mobile phase additive. J Sep Sci 45(11):1847–1855
D’Orazio G (2020) Chiral analysis by nano-liquid chromatography. Trends Analyt Chem 125:115832
Elschner T, Heinze T (2015) Cellulose carbonates: a platform for promising biopolymer derivatives with multifunctional capabilities. Macromol Biosci 15(6):735–746
Elschner T, Ganske K, Heinze T (2013) Synthesis and aminolysis of polysaccharide carbonates. Cellulose 20(1):339–353
Elschner T, Kötteritzsch M, Heinze T (2014) Synthesis of cellulose tricarbonates in 1-butyl‐3‐methylimidazolium chloride/pyridine. Macromol Biosci 14(2):161–165
Engelhardt H, Orth P (1987) Alkoxy silanes for the preparation of silica based stationary phases with bonded polar functional groups. J Liq Chromatogr 10(8–9):1999–2022
Fanali C, D’Orazio G, Gentili A, Fanali S (2019) Analysis of enantiomers in products of food interest. Molecules 24(6):1119
Felix G (2001) Regioselectively modified polysaccharide derivatives as chiral stationary phases in high-performance liquid chromatography. J Chromatogr A 906(1–2):171–184
Ganske K, Heinze T (2018) Evaluation of the synthesis of soluble aromatic cellulose carbonates of low degree of substitution. Macromol Chem Phys 219(15):1800152
Harwood LA, Wong LL, Robertson J (2021) Enzymatic kinetic resolution by addition of oxygen. Angew Chem Int Ed 60(9):4434–4447
Hesse G, Hagel R (1973) Eine vollständige recemattennung durch eluitons-chromagographie an cellulose-tri-acetat. Chromatographia 6(6):277–280
Huang C, Liu J, Fang J, Jia X, Zheng Z, You S, Qin B (2022) Ketoreductase catalyzed (dynamic) kinetic resolution for biomanufacturing of chiral chemicals. Front Bioeng Biotechnol 10:929784
Ichida A, Shibata T, Okamoto I, Yuki Y, Namikoshi H, Toga Y (1984) Resolution of enantiomers by HPLC on cellulose derivatives. Chromatographia 19(1):280–284
Ikai T, Okamoto Y (2009) Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chem Rev 109(11):6077–6101
Jin L, Gao W, Li L, Ye J, Lin C, Liu W (2010) Enantioseparation and enantioselective analysis of chiral herbicides. In: Soloneski S, Laramendy M (eds) Herbicides, Theory Applications. IntechOpen, Rijeka, Croatia
Jusner P, Bausch F, Schiehser S, Schwaiger E, Potthast A, Rosenau T (2022) Protocol for characterizing the molar mass distribution and oxidized functionality profiles of aged transformer papers by gel permeation chromatography (GPC). Cellulose 29(4):2241–2256
Kaida Y, Okamoto Y (1993) Optical resolution on regioselectively carbamoylated cellulose and amylose with 3,5-dimethylphenyl and 3,5-dichlorophenyl isocyanates. Bull Chem Soc Jpn 66(8):2225–2232
Katoh Y, Tsujimoto Y, Yamamoto C, Ikai T, Kamigaito M, Okamoto Y (2011) Chiral recognition ability of cellulose derivatives bearing pyridyl and bipyridyl residues as chiral stationary phases for high-performance liquid chromatography. Polym J 43(1):84–90
Kohout M, Hovorka Å, Herciková J, Wilk M, Sysel P, Izák P, Bartůněk V, von Baeckmann C, Pícha J, Frühauf P (2019) Evaluation of silica from different vendors as the solid support of anion-exchange chiral stationary phases by means of preferential sorption and liquid chromatography. J Sep Sci 42(24):3653–3661
Lao W, Gan J (2006) High-performance liquid chromatographic separation of imidazolinone herbicide enantiomers and their methyl derivatives on polysaccharide-coated chiral stationary phases. J Chromatogr A 1117(2):184–193
Liu RQ, Bai LY, Zhang YJ, Zhang YP (2013) Green synthesis of a typical chiral stationary phase of cellulose tris(3,5-dimethylphenylcarbamate). Chem Cent J 7(1):1–5
Martín-Biosca Y, García‐Ruiz C, Marina ML (2001) Enantiomeric separation of chiral phenoxy acid herbicides by electrokinetic chromatography. Application to the determination of analyte‐selector apparent binding constants for enantiomers. Electrophoresis 22(15):3216–3225
Miaomiao H, Xiaosa J, Han Y, Xiao L, Yadong L, Shengxiang J (2017) Controlled synthesis, immobilization and chiral recognition of carboxylic acid functionalized cellulose tris(3,5-dimethylphenylcarbamate). Carbohydr Polym 172:223–229
Minguillón C, Franco P, Oliveros L, López P (1996) Bonded cellulose-derived high-performance liquid chromatography chiral stationary phases I. Influence of the degree of fixation on selectivity. J Chromatogr A 728(1–2):407–414
Mukherjee A, Bera A (2012) Importance of chirality and chiral chromatography in pharmaceutical industry: a detailed study. J Chem Pharm 2(4):334–346
Musa MM (2020) Enzymatic racemization of alcohols and amines: an approach for bi-enzymatic dynamic kinetic resolution. Chirality 32(2):147–157
Okada Y, Yamamoto C, Kamigaito M, Gao Y, Shen J, Okamoto Y (2016) Enantioseparation using cellulose tris(3,5-dimethylphenylcarbamate) as chiral stationary phase for HPLC: Influence of molecular weight of cellulose. Molecules 21(11):1484
Okamoto Y, Kaida Y (1994) Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J Chromatogr A 666(1–2):403–419
Okamoto Y, Kawashima M, Hatada K (1984) Chromatographic resolution. 7. Useful chiral packing materials for high-performance liquid chromatographic resolution of enantiomers: phenylcarbamates of polysaccharides coated on silica gel. J Am Chem Soc 106(18):5357–5359
Okamoto Y, Kawashima M, Hatada K (1986) Chromatographic resolution: XI. Controlled chiral recognition of cellulose triphenylcarbamate derivatives supported on silica gel. J Chromatogr A 363(2):173–186
Pasteur L (1858) Transformation of the two kinds of tartaric acid into racemic acid. Discovery of inactive tartaric acid. New method of separating racemic acid into the two tartaric acids, right and left. CR Acad Sci 46:615–618
Peng Y, He QS, Cai J (2016) Enantioseparation of citalopram by RP-HPLC, using sulfobutyl ether-β-cyclodextrin as a chiral mobile phase additive. Int J Anal Chem 2016:1–7
Pourjavadi A, Seidi F, Afjeh SS, Nikoseresht N, Salimi H, Nemati N (2011) Synthesis of soluble N-functionalized polysaccharide derivatives using phenyl carbonate precursor and their application as catalysts. Starch‐Stärke 63(12):780–791
Qian D, Bera S, Hu X (2021) Chiral alkyl amine synthesis via catalytic enantioselective hydroalkylation of enecarbamates. J Am Chem Soc 143(4):1959–1967
Qin Q, Zhang S, Zhang WG, Zhang ZB, Xiong YJ, Guo ZY, Fan J, Run-Zheng S, Finlow D, Yin Y (2010) The impact of silica gel pore and particle sizes on HPLC column efficiency and resolution for an immobilized, cyclodextrin‐based, chiral stationary phase. J Sep Sci 33(17–18):2582–2589
Rocco A, Aturki Z, Fanali S (2013) Chiral separations in food analysis. J Agric Food Chem 52:206–225
Salon MCB, Gerbaud G, Abdelmouleh M, Bruzzese C, Boufi S, Belgacem MN (2007) Studies of interactions between silane coupling agents and cellulose fibers with liquid and solid-state NMR. Magn Reson Chem 45(6):473–483
Shen J, Okamoto Y (2016) Efficient separation of enantiomers using stereoregular chiral polymers. Chem Rev 116(3):1094–1138
Shen J, Wang F, Bi W, Liu B, Liu S, Okamoto Y (2018) Synthesis of cellulose carbamates bearing regioselective substituents at 2,3- and 6-positions for efficient chromatographic enantioseparation. J Chromatogr A 1572:54–61
Singh M, Sethi S, Bhushan R (2020) Liquid chromatographic methods for separation, determination, and bioassay of enantiomers of etodolac: a review. J Sep Sci 43(1):18–30
Spelmezan CG, Bencze LC, Katona G, Irimie FD, Paizs C, Toșa ML (2020) Efficient and stable magnetic chitosan-lipase B from Candida antarctica bioconjugates in the enzymatic kinetic resolution of racemic heteroarylethanols. Molecules 25(2):350
Subramanian G (2008) Chiral separation techniques: a practical approach. John Wiley & Sons, Weinheim, Germany
Sun C, Li Y, Yin G (2022) Practical synthesis of chiral allylboronates by asymmetric 1,1-difunctionalization of terminal alkenes. Angew Chem Int Ed 61(37):e202209076
Tang S, Ikai T, Tsuji M, Okamoto Y (2010) Immobilization of 3,5-dimethylphenylcarbamates of cellulose and amylose onto silica gel using (3‐glycidoxypropyl) triethoxysilane as linker. J Sep Sci 33(9):1255–1263
Tang S, Liu G, Li X, Jin Z, Wang F, Pan F, Okamoto Y (2011) Improved preparation of chiral stationary phases via immobilization of polysaccharide derivative-based selectors using diisocyanates. J Sep Sci 34(15):1763–1771
Wei Q, Su H, Gao D, Wang S (2019) HPLC with cellulose tris(3,5-dimethylphenyl carbamate) chiral stationary phase: Influence of coating times and coating amount on chiral discrimination. Chirality 31(3):164–173
Wojaczyńska E, Wojaczyński J (2020) Modern stereoselective synthesis of chiral sulfinyl compounds. Chem Rev 120(10):4578–4611
Xue YP, Cao CH, Zheng YG (2018) Enzymatic asymmetric synthesis of chiral amino acids. Chem Soc Rev 47(4):1516–1561
Yang LC, Deng H, Renata H (2022) Recent progress and developments in chemoenzymatic and biocatalytic dynamic kinetic resolution. Org. Process Res Dev 26(7):1925–1943
Yashima E, Sahavattanapong P, Okamoto Y (1996) HPLC enantioseparation on cellulose tris(3,5-dimethylphenylcarbamate) as a chiral stationary phase: influences of pore size of silica gel, coating amount, coating solvent, and column temperature on chiral discrimination. Chirality 8(6):446–451
Yin C, Chen W, Zhang J, Zhang M, Zhang J (2019a) A facile and efficient method to fabricate high-resolution immobilized cellulose-based chiral stationary phases via thiol-ene click chemistry. Sep Purif Technol 210:175–181
Yin C, Zhang J, Chang L, Zhang M, Yang T, Zhang X, Zhang J (2019b) Regioselectively substituted cellulose mixed esters synthesized by two-steps route to understand chiral recognition mechanism and fabricate high-performance chiral stationary phases. Anal Chim Acta 1073:90–98
Ying Z, Ling L, Kunde L, Xinping Z, Weiping L (2009) Enantiomer separation of triazole fungicides by high-performance liquid chromatography. Chirality 21(4):421–427
Yu L, Wang S, Zeng S (2019) Chiral mobile-phase additives in HPLC enantioseparations. In: Scriba GKE (ed) Chiral Separations. Springer, Humana New York, USA
Zhang GH, Xi JB, Chen W, Bai ZW (2020) Comparison in enantioseparation performance of chiral stationary phases prepared from chitosans of different sources and molecular weights. J Chromatogr A:461029
Zhang T, Francotte E (1995) Chromatographic properties of composite chiral stationary phases based on cellulose derivatives. Chirality 7(6):425–433
Zhao P, Dong X, Chen X, Guo X, Zhao L (2019) Stereoselective analysis of chiral pyrethroid insecticides tetramethrin and α-cypermethrin in fruits, vegetables, and cereals. J Agric Food Chem 67(33):9362–9370
Zheng J, Bragg W, Hou J, Lin N, Chandrasekaran S, Shamsi SA (2009) Sulfated and sulfonated polysaccharide as chiral stationary phases for capillary electrochromatography and capillary electrochromatography–mass spectrometry. J Chromatogr A 1216(5):857–872
Zhu C, Cai Y, Jiang H (2021) Recent advances for the synthesis of chiral sulfones with the sulfone moiety directly connected to the chiral center. Org Chem Front 8(19):5574–5589
Acknowledgments
The authors would like to thank the University of Natural Resources and Life Sciences, Vienna (BOKU) and the County of Lower Austria for their financial support in the framework of the “Austrian Biorefinery Center Tulln” (ABCT) and the BOKU doctoral school “Advanced Biorefineries: Chemistry & Materials” (ABC&M). C.V.B. is grateful for an Ernst Mach Grant (ASEA-UNINET, ICM-2019-13801). The financial support by the Gesellschaft für Forschungsförderung Niederösterreich m.b.H. (H.H., project LSC20-002) is gratefully acknowledged. Peter Frühauf (University of Vienna) is gratefully acknowledged for his support in column packing. Open access funding is provided by the University of Natural Resources and Life Sciences, Vienna (BOKU). Assoc.Prof. Dr. Michal Kohout (University of Chemistry and Technology, Prague, Czech Republic) is acknowledged for providing chiral analytes.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The authors would like to thank the University of Natural Resources and Life Sciences, Vienna (BOKU) and the County of Lower Austria for their financial support in the framework of the “Austrian Biorefinery Center Tulln” (ABCT) and the BOKU doctoral school “Advanced Biorefineries: Chemistry & Materials” (ABC&M). C.V.B. is grateful for an Ernst Mach Grant (ASEA-UNINET, ICM-2019-13801). The financial support by the Gesellschaft für Forschungsförderung Niederösterreich m.b.H. (H.H., project LSC20-002) is gratefully acknowledged. Open access funding is provided by the University of Natural Resources and Life Sciences, Vienna (BOKU).
Author information
Authors and Affiliations
Contributions
C.V.B, T.R. and H.H. contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.V.B. The original draft of the manuscript was written by C.V.B, including visualization. Review & editing by T.R. and H.H. Supervision, project administration and funding acquisition by T.R. and H.H. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors agreed to the publication in the submitted form.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bui, C.V., Rosenau, T. & Hettegger, H. Synthesis by carbonate aminolysis and chiral recognition ability of cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(α-phenylethyl carbamate) selectors. Cellulose 30, 153–168 (2023). https://doi.org/10.1007/s10570-022-04898-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04898-8