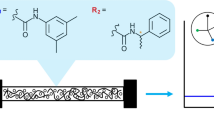
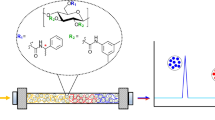
Abstract
A new synthesis strategy for the preparation of cellulose derivative-based chiral selectors and the subsequent mild immobilization onto pre-functionalized silica gel are introduced, utilizing Cu(I)-catalyzed alkyne-azide Huisgen cycloaddition (“click”) chemistry. A cellulose 3,5-dimethylphenyl carbamate derivative carrying propynyl carbamate groups was prepared by a combination of carbonate aminolysis and isocyanate chemistry. For immobilization, 3-azidopropyl-functionalized silica gel as an inert carrier was used, synthesized via a 3-chloropropyl intermediate. The chiral selector, as well as the inorganic/organic hybrid materials (silica gel/chiral selector), were comprehensively characterized by ATR-FTIR, solid-state 13C and 29Si NMR, liquid-state NMR, GPC, TGA, and elemental analysis. The enantioseparation performance of the immobilized-type chiral stationary phase was evaluated by HPLC with a set of representative chiral test analytes and different eluents and compared to a respective coated-type (=non-covalently bound) chiral stationary phase carrying the same selector quality and quantity on the same silica gel matrix. The immobilization did not adversely affect the chiral separation performance; on the contrary, in some chromatographic separations the immobilized-type chiral stationary phase surprisingly even surpassed the coated reference material.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cellulose is a well-known raw material in many industrial applications, e.g., paper, board, fiber production, and various cellulose derivatives. Besides this large-scale (and comparably low value) utilization, there are also various niche applications, in which cellulose is processed into specialized high-tech and high-value materials on a lower scale. Cellulose derivative-based chiral selectors (CSs), key components in chromatographic columns for enantioseparation applications in liquid chromatography (besides amylose derivatives), are one example. Natural cellulose—which is per se chiral and enantiomerically pure—is used as a CS, however, the enantioseparation performance does not meet the requirements of analytical and preparative scale separation. Therefore, cellulose needs to be derivatized to increase the overall enantioseparation performance (Li et al. 2020; Wang et al. 2021a). The enantioseparation ability of cellulose derivatives was originally investigated by Lüttringhaus and co-workers in 1967 when they applied cellulose triacetate for the enantioseparation of 4.5.6.7-dibenzo-1.2-dithiacyclooctadien in liquid chromatography (Lüttringhaus et al. 1967). A large number of CSs based on phenyl carbamates and/or benzoate esters of cellulose was introduced and has been commercialized over the last 40–50 years (Minguillón et al. 1996; Fanali et al. 2019; Onishi et al. 2022). Polysaccharide derivative-based CSs based on cellulose and amylose can nowadays separate about 90% of the tested racemic compounds and enantiomeric mixtures (Ikai and Okamoto 2009; Shen and Okamoto 2016; Yin et al. 2019). Important fields of enantioseparation are, inter alia, stereoselective synthesis (Chebrouk et al. 2019; D’Orazio 2020; Shi et al. 2020), the analysis of enantiomers in food products (Fanali et al. 2019; Alvarez-Rivera et al. 2020), fungicides (Ying et al. 2009; Ruiz-Rodríguez et al. 2015; Wang et al. 2021b), herbicides (Martín‐Biosca et al. 2001; Lao and Gan 2006; Jin et al. 2010), insecticides (Zhang et al. 2016; Zhao et al. 2019), pesticides (Carrão et al. 2019; Merino et al. 2019; Li et al. 2022), and—very importantly—pharmaceutical products (Mukherjee and Bera 2012; Yaling and Alex 2013; Singh et al. 2020).
High-performance liquid chromatography (HPLC) is one of the most powerful techniques for direct enantioseparation on both analytical and preparative scales (Ahuja 1997; Subramanian 2008). In the case of HPLC (including its variant supercritical fluid chromatography, SFC), a chiral stationary phase (CSP) composed of silica gel as an inert, mechanically stable carrier is combined with an enantiopure CS and used for the direct separation of enantiomers via the formation of differently stable transient diastereomeric complexes between CS and the respective enantiomers (Davankov 1997; Lämmerhofer 2010). The conventional method for the preparation of CSPs is the physical coating of CSs onto (often pre-functionalized) silica gel. Pioneering work in this field has been performed by Okamoto et al. since the 1980s (Okamoto et al. 1984b), from which many coated-type CSPs resulted that have been successfully commercialized and are still available (Okamoto et al. 1984a, 1986; Okamoto and Kaida 1994; Yamamoto and Okamoto 2004; Yin et al. 2019; Bui et al. 2021). However, the high solubility and the swelling behavior of polysaccharide derivative-based CSs limits their use, with a number of organic solvents or mobile-phase components, such as chloroform, tetrahydrofuran, ethyl acetate, acetone, etc., being rather incompatible. This leaves comparably little room for method optimization and development due to the limited number of applicable solvents, narrowing down the choices of chromatographic mobile phases (Minguillón et al. 1996; Yashima et al. 1998; Francotte 2001; Ikai et al. 2007b; Padró and Keunchkarian 2018; Chankvetadze 2019; Yin et al. 2019; Wang et al. 2021a; Onishi et al. 2022).
Immobilization of CSPs, i.e., covalent chemical linkage of cellulose derivative-based CSs onto pre-functionalized silica gel, is thus the most obvious and suitable option to overcome the shortcomings of its “coated-only” counterparts, and their inherent problems with the dissolution of the CS in strong eluents, resulting column bleeding, and, in the worst case, destruction of the column material (Tang et al. 2011; Padró and Keunchkarian 2018; Chankvetadze 2019; Fernandes et al. 2021; Onishi et al. 2022). One of the first immobilized, chemically robust CSPs were introduced by Okamoto et al. as well, when they linked cellulose 3,5-dimethylphenyl carbamate- and 3,5-dichlorophenyl carbamate-type selectors onto 3-aminopropyl-functionalized silica gel by crosslinking with diisocyanates (Okamoto et al. 1987). However, immobilized CSPs are often characterized by a somewhat lower enantiomer recognition capacity compared to their coated-type counterparts, due to reduced flexibility of the CS, especially when having many anchor points to the supporting matrix along the polymer chain (Chang et al. 2018).
Several methods to immobilize CSs onto pre-functionalized silica gels have been introduced: linkage/crosslinking with diisocyanates (Chen et al. 2002, 2003a, b, c; Tang et al. 2011), condensation of alkoxysilyl groups (Ikai et al. 2006, 2007a; Tang et al. 2010a, b; Shen et al. 2012; Yu et al. 2020), polymerization with vinyl groups (Kimata et al. 1993; Minguillón et al. 1996; Franco et al. 1998; Garcés et al. 2003; Kubota et al. 2004; Chen et al. 2006; Chen and Hsieh 2011; Bae et al. 2011), epoxy groups (Chankvetadze et al. 2004; Dong et al. 2008), amidation (Miaomiao et al. 2017), thiol-ene addition (Huang et al. 2014; Yao et al. 2016; Yin et al. 2019; Li et al. 2019; Zhou et al. 2020), and Staudinger ligation (Zhang et al. 2007; Peng et al. 2012; Tan et al. 2014; Silva et al. 2017; Lin et al. 2018). As self-crosslinking reactions are often hard to control, the immobilization of polysaccharide-type CSs by diisocyanate, alkoxysilyl, and vinyl group methods is challenging, and the corresponding immobilized-type CSPs have typically a lower chiral discrimination capacity in comparison to the respective coated CSPs. Further possible drawbacks associated with the above-mentioned immobilization methods are the decrease in chiral discrimination capacity, low immobilization efficiency, low stability of the linkage between CS and pre-functionalized silica gel, as well as time-consuming and complex chemical processes and reactions in the preparation of the immobilized-type CSPs (Kubota et al. 2004).
The jargon term click chemistry introduced by Sharpless and colleagues in 2001 (Kolb et al. 2001) is usually referring to fast, neat, and easy-to-handle chemical reactions, which are efficiently carried out under mild conditions with almost quantitative yield. The most well-known example is the Cu(I)-catalyzed alkyne-azide Huisgen cycloaddition, in which stable, mostly 1,4-disubstituted 1,2,3-triazoles are formed (Rostovtsev et al. 2002; Aflak et al. 2019). Nowadays, this reaction is applied in many technological areas, such as drug discovery (Totobenazara and Burke 2015), material sciences, chemical biology (Lauria et al. 2014; Burke and Marques 2015), cell labeling (Sletten and Bertozzi 2011; Burke and Marques 2015), and peptidomimetic chemistry (Pedersen and Abell 2011; He et al. 2014; Solum et al. 2014; Pingaew et al. 2014), to name but a few. The alkyne-azide cycloaddition reaction has already been used for the immobilization of Cinchona derivatives (bearing alkyne groups) onto 3-azidopropyl-functionalized silica gel to prepare anion-exchange-type CSPs (Kacprzak et al. 2006, 2011; Kacprzak and Lindner 2011; Hettegger et al. 2014). In cellulose chemistry, this type of click chemistry is also well-developed. The polysaccharide can either act as the alkyne donor (for instance after etherification with propargyl halides) or as the azido-type acceptor (e.g., after tosylation and exchange for azide at C6 or after heterogeneous functionalization with (3-azidopropyl)trialkoxysilanes) (Liebert et al. 2006; Hettegger et al. 2015a, 2015b, 2016).
In this account, we would like to communicate another approach, a one-pot, three-step synthesis of a cellulose 3,5-dimethylphenyl carbamate-type selector carrying propynyl carbamate groups for click immobilization. This is achieved by a combination of carbonate aminolysis and isocyanate chemistry. The obtained cellulose derivatives were subsequently immobilized onto 3-azidopropyl-functionalized silica gel by the mentioned alkyne-azide Huisgen cycloaddition chemistry to give chemically and physically robust CSPs. All compounds and materials were comprehensively analytically characterized by FTIR, solid-state 13C and 29Si NMR, 2D liquid-state NMR, GPC, and elemental analysis (EA). The loading amount of the CS on the silica matrix (i.e., the organic content of the immobilized CSP) was determined by both EA and TGA. Eventually, the enantioseparation performance of the immobilized-type CSP was evaluated and compared to a coated-type CSP prepared with the same CS type and CS amount.
Materials and methods
Materials
Microcrystalline cellulose (Avicel® PH-101), N,N-diisopropylethylamine (> 99%), p-toluenesulfonic acid (98.5%), tetra-n-butylammonium iodide (99%), and p-cymene (99%) were purchased from Sigma-Aldrich (Schnelldorf, Germany). Microcrystalline cellulose was dried at 40 °C in a vacuum oven for at least two days before use. 3,5-Dimethylphenyl isocyanate (> 98%), phenyl chloroformate (> 98%), (3-chloropropyl)trimethoxysilane (> 97%), and NaN3 (> 99%) were purchased from TCI Europe N.V. (Zwijndrecht, Belgium). Organic solvents, such as N,N-dimethylacetamide (DMAc), N,N-dimethylformamide (DMF), tetrahydrofuran (THF), acetonitrile (MeCN), and pyridine, were all reagent grade and dried over 3 Å molecular sieves (Sigma-Aldrich) for at least three days before use. Ethanol (EtOH) and methanol (MeOH) for precipitation and washing were of technical grade and obtained from Carl Roth GmbH + Co. KG (Karlsruhe, Germany) or Fisher Scientific (Vienna, Austria). Silica gel (NUCLEOSIL® 1000–7, 7 μm, 1000 Å, 25 m2/g by BET) was purchased from Bruckner Analysentechnik GmbH (Linz, Austria). Rhodamine B propargyl ester was synthesized according to a published protocol (Hettegger et al. 2016). Empty stainless steel HPLC columns (150 × 4 mm, i.d.) and column hardware were purchased from Bischoff Analysentechnik u. -geräte GmbH (Leonberg, Germany). The commercial chiral analytes, 2-phenylcyclohexanone (> 98%, a), benzoin (> 98%, b), and Pirkle's alcohol (> 99%, d), were purchased from TCI Europe N.V. (Zwijndrecht, Belgium). Flavanone (98%, c), trans-stilbene oxide (98%, e), and Tröger's base (98%, f) were obtained from Sigma-Aldrich (Schnelldorf, Germany). Mandelic acid derivatives (g–j), 1-methoxy-2-(1-methoxy-3-phenylpropyl) benzene (k), and 1-(o-hydroxyphenyl)-3-phenyl-1-propanol (l) were synthesized according to standard procedures. The HPLC solvents n-hexane (95%, n-hex), 2-propanol (99.9%, IPA), tetrahydrofuran (> 99.9%, THF), and chloroform (99.8%) were obtained from Fisher Scientific. Formic acid (97.5–98.5%, FA) and diethylamine (99.5%, DEA) as modifiers for the HPLC solvents were purchased from Sigma-Aldrich (Schnelldorf, Germany).
Instrumentation
Elemental analyses were performed with a EURO EA 3000 CHNS-O instrument (HEKAtech, Wegberg, Germany), and halide contents were determined by argentometry, both at the microanalytical laboratory of the University of Vienna, Austria. Thermogravimetric analysis (TGA) was carried out with a TG 209 F1 Iris thermo-microbalance (Netzsch GmbH & Co. KG, Selb, Germany), with a dried sample mass of 10–15 mg, an oxidizing atmosphere (N2:O2 = 4:1, v/v), a flow rate of 20 mL/min and a T gradient of 10 °C/min. All measurements were done in triplicate. Proteus software was used for data processing and evaluation of TGA results. Solid-state 13C CP/MAS (12 kHz), 29Si CP/MAS (8 kHz), and HSQC liquid-state NMR experiments were carried out on Avance III HD and Avance II 400 instruments (Bruker, Rheinstetten, Germany) with a resonance frequency of 400.13 MHz for 1H, 100.67 MHz for 13C and 79.53 MHz for 29Si. NMR spectra were evaluated using both TopSpin 3.6.2 and ACD/NMR Processor Academic Edition 12.01. Chemical shifts (δ) are given in ppm. ATR-FTIR spectra were recorded on a Frontier IR single-range spectrometer (PerkinElmer, Waltham, Massachusetts, US) equipped with a diamond/ZnSe crystal, LiTaO3 detector, and KBr windows. Data processing was performed with SpectraGryph software (version v1.2.15). GPC analyses were performed according to standard procedures. For details about GPC procedures see (Henniges et al. 2011; Jusner et al. 2022). An Agilent Technologies, Inc. (Santa Clara, CA, USA) 1100 HPLC apparatus equipped with a degasser (G1322A), quaternary pump (G1311A), autosampler (G1313A), thermostatted column compartment (G1316A), and DAD (G1315A) was used to evaluate the enantioseparation performance of the chiral columns. OpenLab CDS software (Agilent) was used for chromatography data processing and evaluation.
Synthesis
One-pot, three-step synthesis of the chiral selector (CS)
-
(1)
Oxycarbonylation: Microcrystalline cellulose (1.5 g) was immersed in anhydrous DMAc (45 mL) and then heated at 120 °C for 2 h under a dry nitrogen atmosphere during vigorous stirring. Cellulose degradation during that heating period was considered to be tolerable (Potthast et al. 2003). Anhydrous LiCl (2.7 g) was added slowly after cooling down to < 90 °C. The mixture was allowed to stir at room temperature (RT) until a clear solution was formed. Anhydrous pyridine (4.5 mL) was added slowly and the homogeneous solution was cooled down to 0 °C by an ice/water bath. Phenyl chloroformate (0.36 mL, 0.3 molar equivalents with respect to the glucopyranose repeating unit of cellulose) was added dropwise during 10 min and the mixture was stirred for 12 h at RT.
-
(2)
Aminolysis: Anhydrous DMF (4.5 mL) was added to the reaction mixture, followed by the addition of propargylamine (0.78 mL, 5 molar equivalents with respect to phenyl chloroformate), and the mixture was stirred at 40 °C for 12 h.
-
(3)
Carbamoylation: Anhydrous pyridine (15 mL) was slowly added to the reaction mixture, followed by 3,5-dimethylphenyl isocyanate (5.5 mL, 4.5 molar equivalents rel. to the glucopyranose units of cellulose). The mixture was stirred at 80 °C for 18 h and subsequently cooled down to RT. A large excess of distilled water was used to precipitate the crude CS, which was collected by vacuum filtration, and washed with a large excess of distilled water and EtOH (two times each). The crude CS was dried at 40 °C in a vacuum oven for two days and re-dissolved in acetone (375 mL). Residual LiCl was separated by vacuum filtration through a sintered frit. Acetone was largely removed by rotary evaporation under reduced pressure and the CS was precipitated in EtOH, collected by vacuum filtration, washed with a large excess of EtOH and distilled water (two times each), and dried at 40 °C in a vacuum oven for two days. Yield: 4.85 g, 87 wt%.
Pre-modification of silica gel (AzPS)
Protocols reported by (Kacprzak et al. 2006) and (Hettegger et al. 2014) were adapted to synthesize 3-azidopropyl-functionalized silica gel (AzPS) with modifications.
-
(1)
3-Chloropropyl-functionalized silica gel: Silica gel (10 g) and p-toluenesulfonic acid (20 mg) as a catalyst were immersed in toluene (200 mL) and mechanically stirred. The suspension was dried by azeotropic distillation under a dry nitrogen atmosphere. After distilling off about half of the volume of toluene, the temperature of the suspension was reduced to 80 °C, and (3-chloropropyl)trimethoxysilane (4.6 mL, 25.2 mmol) was added dropwise to the mixture during 15 min. The reaction mixture was mechanically stirred for 48 h. The suspension was then cooled down to RT and the formed ClPS was collected by vacuum filtration through a sintered glass frit (DURAN®, porosity 4), washed with toluene (100 mL), and with MeOH (100 mL, two times each). ClPS was subsequently re-immersed in toluene (100 mL) and mechanically stirred at 80 °C for 2 h. The suspension was then cooled down to RT and ClPS was again collected by vacuum filtration, washed with toluene (100 mL), MeOH (100 mL), and distilled water (200 mL, two times each), and dried at 40 °C in a vacuum oven for two days. Yield: 10.0 g.
-
(2)
3-Azidopropyl-functionalized silica gel: ClPS (10 g), tetra-n-butylammonium iodide (60 mg) as a catalyst, and NaN3 (4.9 g, 75.4 mmol) were dispersed in DMSO (100 mL) and the suspension was mechanically stirred at 80 °C for 72 h under a dry nitrogen atmosphere and then cooled to RT. The obtained AzPS was collected by vacuum filtration through a sintered glass frit (DURAN®, porosity 4), washed with H2O (500 mL) and MeOH (250 mL, two times each), and dried at 40 °C in a vacuum oven for two days. Yield: 10.0 g.
Preparation of immobilized CS (CSP1)
The above CS (1 g) was dissolved and stirred in THF (100 mL) in a Schott DURAN® bottle. AzPS (3 g) was added and the suspension was shaken on an overhead shaker for 24 h. A catalyst solution was prepared by dissolving CuI (17 mg) in MeCN (10 mL). N,N-Diisopropylethylamine (1 mL) was added to the CS / AzPS mixture in THF, followed by the CuI solution. The mixture was degassed by purging with N2 through a syringe needle for 30 min. The vessel was closed, sealed with Parafilm®, and allowed to slowly rotate for 24 h on an overhead shaker at RT. CSP1 was collected by vacuum filtration through a sintered glass frit (DURAN®, porosity 4), washed with THF (200 mL), MeCN (100 mL), MeOH (100 mL), and distilled water (200 mL, two times each), and dried at 40 °C in a vacuum oven for two days. The modified silica particles were sieved through an analytical sieve (40 μm mesh) before being used in column packing. Yield: 2.67 g, 84 wt%.
Preparation of coated CS (CSP2)
The above CS (0.192 g) was dissolved in THF (40 mL) and the solution was transferred to a round-bottomed flask that contained AzPS (3.0 g; coating amount = 6.0 wt%). The mixture was sonicated for 20 min in an ultrasonic bath. THF was slowly evaporated at 40 °C and 357 mbar in a rotary evaporator. After evaporation and drying in a vacuum oven at 40 °C overnight, the coated silica particles were sieved through an analytical sieve (40 μm mesh) before being used in column packing. Yield: 2.79 g, 87 wt%.
Column packing
Each CSP (2.3 g) was immersed in a mixture of IPA (20 mL) and acetic acid (100 µL). The suspension was sonicated in an ultrasonic bath for 20 min to form a homogeneous slurry, which was then introduced under high pressure (max. 290 bar) into an empty stainless steel HPLC column in an in-house column-packing apparatus. MeOH was used (approx. 120 mL) as the compacting agent. After packing, the columns were rinsed with IPA in a standard HPLC setup (flow rate: 1.0 mL/min) and stored until further use.
HPLC method
Before use, the CSP1 and CSP2 columns were rinsed with n-hex/IPA using a stepwise gradient (30:70, 60:40, 90:10, v/v). The concentration of all analytes was 1 mg/mL. The flow rate and injection volume were set to 1 mL/min and 5 μL, respectively. The absorbance of all analytes was recorded at 254 nm. p-Cymene was used to determine the dead time (t0) of the system. All measurements were carried out in triplicate.
Results and discussion
One-pot, three-step synthesis of the CS
Cellulose 3,5-dimethylphenyl carbamate containing propynyl carbamate substituents as a chemical anchor for subsequent click chemical immobilization to pre-functionalized AzPS was synthesized in three steps in one pot by applying both carbonate aminolysis and isocyanate chemistry (Fig. 1).
(A) One-pot, three-step synthesis of the cellulose 3,5-dimethylphenyl carbamate-type CS carrying propynyl carbamate anchor groups; conditions: (a) DMAc/LiCl/pyridine, 0 °C to RT, N2, 12 h; (b) DMAc/LiCl/pyridine/DMF, 40 °C, N2, 12 h; (c) DMAc/LiCl/pyridine/DMF, 80 °C, N2, 18 h; (B) pre-functionalization of silica gel for obtaining AzPS via ClPS; conditions: (d) toluene, p-toluenesulfonic acid (cat.), 80 °C, N2, 48 h; (e) DMSO, tetra-n-butylammonium iodide (cat.), 80 °C, N2, 72 h; (C) chemical structure of the 1,2,3-triazole linker after heterogeneous click chemical immobilization of the CS onto AzPS yielding CSP1
Cellulose was homogeneously dissolved in DMAc/LiCl according to a protocol by Heinze et al. (Pourjavadi et al. 2011; Elschner et al. 2013; Ganske and Heinze 2018). After the addition of pyridine, phenyl chloroformate was added (0.3 molar eq.). The higher reactivity and selectivity of phenyl chloroformate towards C6-OH versus C2-OH and C3-OH have already been reported (Elschner et al. 2014; Ganske and Heinze 2018). In a previous study, we have optimized the protocol towards quantitative oxycarbonylation of C6-OH (Bui et al. 2022b) and also reported on the optimization and successful aminolysis reaction between the polysaccharidic carbonates (Bui et al. 2022a). The obtained phenyl carbonate served as a reactive intermediate for subsequent aminolysis with propargylamine yielding a propynyl carbamate moiety as an alkyne donor for immobilization of the CS onto 3-azidopropyl-functionalized silica gel (Fig. 1C). After aminolysis, the residual OH groups were reacted directly, in the same pot, with 3,5-dimethylphenyl isocyanate for obtaining a “classical” cellulose 3,5-dimethylphenyl carbamate-type CS. The degrees of substitution of propynyl carbamate and 3,5-dimethylphenyl carbamate substituents calculated based on EA results (Supplementary Information) were 0.14 and 2.82, respectively. The yield of derivatization was 98.7%.
The FTIR, HSQC liquid-state NMR and solid-state 13C NMR spectra of the CS are shown in Figs. S1, S2, and Fig. 2, respectively. The EA results for the CS are given in Table 1. In the FTIR spectrum of the CS (see Fig. S1), the bands assigned to N–H groups at 3321, C=O groups at 1720, C=C aromatic ring signals at 1611, 1539, 1450, 839, and C–O at 1212 cm−1 indicated that the carbamoylation reaction was successful, with the product fully matching the literature data of cellulose 3,5-dimethylphenyl carbamate-type selectors (Liu et al. 2013; Wei et al. 2019). In the solid-state 13C NMR spectrum of CS, the resonances assigned to carbamate C=O groups at 152.6, C=C aromatic ring carbons at 138.3, 125.0, and 116.1, as well as CH3-Ph at 20.8 ppm support the successful carbamoylation (Fig. 2).
The presence of resonances assigned to C8 at 2.7/31.9 ppm and C9 at 6.1/84.2 ppm (1H/13C, see the solution HSQC NMR spectra in Fig. S2) proved also the presence of propynyl groups on the polysaccharide backbone of the CS. Note that these resonances were not visible in the solid-state 13C NMR spectrum due to the low propargyl carbamate DS. The molecular weight, dispersity (Đ), and calculated DP of CS were 210 kDa, 2.4, and 347, respectively.
Pre-modification of silica gel
3-Chloropropyl-functionalized silica gel (ClPS) was synthesized as an intermediate by condensation of (3-chloropropyl)trimethoxysilane with neat silica gel in toluene. The 3-chloropropyl-modified silica gel was subsequently converted to 3-azidopropyl-functionalized silica gel as the actual carrier material by nucleophilic substitution with NaN3 in DMSO (Fig. 1B). The synthesis and the chemical structure of ClPS were evaluated by solid-state 29Si and 13C NMR. The EA results are summarized in Table 1. The different types of condensation of trialkoxysilanes onto silica gel and their resonances in solid-state 29Si NMR according to (Salon et al. 2007) are illustrated in Fig. S3. The solid-state 29Si NMR spectra of ClPS versus neat silica gel and the solid-state 13C NMR spectrum of ClPS are shown in Figs. S4 and S5, respectively. The additional resonances in the 29Si NMR spectrum assigned to T1 at approx. -50 ppm, T2 at approx. -60 ppm, and T3 at approx. -70 ppm and the resonances of Cγ at 45.5, Cβ at 26.1, and Cα at 9.7 ppm in the 13C NMR spectrum of ClPS indicated successful fixation of 3-chloropropyl groups on the silica matrix. The loading of 3-chloropropyl groups was calculated to be 0.43 wt% based on EA (corresponding to 48 µmol/g silica).
The chemical structure of AzPS was characterized by the above-mentioned solid-state NMR methods as well (Fig. 3). In addition, rhodamine B propargyl ester was used as a color indicator to further confirm the presence of azido-groups and their reactivity in click reactions (see Figs. S6 and S7).
The solid-state 29Si NMR spectrum of AzPS showed resonances assigned to T2 structures at -60 ppm and T3 at -70 ppm, and resonances of Cγ at 53.4 (45.5 ppm for ClPS), Cβ at 22.4 (26.1 ppm for ClPS), and Cα at 9.3 ppm in the 13C NMR spectrum. In addition, the purple-to-pink color of the azido-modified silica gel “clicked” with rhodamine B propargyl ester further proved the presence of azido groups in a qualitative manner. The color test was used as an indirect proof since in ATR-FTIR no azide bands were visible due to their low concentration (33 µmol/g based on an N content of EA).
Click immobilization of CS onto AzPS
The obtained CS carrying propynyl carbamate groups at a low level of substitution (DS = 0.14) was chemically linked to pre-modified silica gel by Cu(I)-catalyzed Huisgen alkyne-azide cycloaddition under mild conditions. The loading amount of immobilized CS on CSP1 was determined by EA (see Table 1) and TGA (see Fig. S8). The total organic content of CSP1 based on EA was 6 wt% (O content estimated based on the oxygen content of the selector). The organic contents of pure silica gel, AzPS, and CSP1 based on the mass change by TGA were approx. 0.6, 1.1, and 7.0 wt%, respectively (see Fig. S8). Thus, the results of both methods agreed very well. To increase the loading amount of CS on the silica gel, we tried to increase the anchor points on both CS (more propargyl groups) and AzPS (higher azide loading), however, the organic content of the corresponding CSP still did not exceed 7.4 wt% (TGA). Due to the low surface area of the silica gel used in this study (7 µm particle size, 1000 Å pore size, 25 m2/g surface area), it thus seemed difficult to achieve a higher loading degree. Higher loading amounts can be achieved using silica gel with a higher surface area (ongoing work).
For comparative purposes, AzPS was also physically coated with CS with the same loading amount (6 wt%).
Enantioseparation evaluation
Twelve chiral analytes (2-phenyl cyclohexanone (a), benzoin (b), flavanone (c), Pirkle's alcohol (d), trans-stilbene oxide (e), Tröger's base (f), four mandelic acid derivatives (g-j), 1-methoxy-2-(1-methoxy-3-phenylpropyl) benzene (k), and 1-(o-hydroxyphenyl)-3-phenyl-1-propanol (l), see Fig. 4) were used in this work to study the enantioseparation performance. We compared the immobilized selector CSP1 and the coated-only alternative CSP2, both with a CS loading of 6 wt% and the same pre-modified silica gel for ensuring a fair comparison. A “standard” mobile phase composed of n-hexane and IPA (9:1, v/v) was used. Furthermore, also “non-standard” mobile phases containing THF and CHCl3 were employed to study the enantioseparation performance and evaluate the stability of the immobilized-type CSP1. The reproducibility of the enantioseparation performance of CSP1 had been examined once per month over five months. The material proved to be perfectly stable. There was only a minute difference found (e.g., RSD = 3.5% for compound e in n-hex:IPA:CHCl3, 87:10:3, v/v/v). Before the tests, CSP1 had been additionally rinsed with a large excess of THF (in which the selector is soluble), MeCN, MeOH and water to ensure that only covalently linked selector is left at the CSP and any contributions of adsorbed CS can be excluded.
Different mobile phases were used for the comparative studies: n-hex/IPA (90:10, v/v), n-hex/IPA/FA (90:10:1, v/v/v), and n-hex/IPA/DEA (90:10:1, v/v/v). The chromatographic retention factor (k1), selectivity (α), and resolution (Rs) of the twelve chiral analytes on the two CSPs are summarized in Tables S1–3. Figure 5 shows the resolution (Rs) values of the chiral analytes on CSP1 and CSP2. Representative HPLC chromatograms of the analytes b and d are shown in Fig. 6. In the “standard” solvent n-hex/IPA (90:10, v/v), all of the selected chiral analytes were at least partially separated on CSP1, except racemate l. The chiral analyte d was the only baseline-separated compound on CSP1 with Rs = 1.68 and α = 1.93. On the other hand, none of the selected chiral analytes were separated on CSP2 using this solvent mixture, except chiral analyte d, which was at least partially separated with Rs = 0.73 and α = 1.86. This demonstrated a clear performance advantage of CSP1 over CSP2 at this comparably low overall selector loading.
HPLC chromatogram of (A) chiral analyte b and (B) compound d on CSP1 and CSP2 using n-hex:IPA (90:10, v/v) as the mobile phase; (C) chiral analyte i on CSP1 with n-hex:IPA:THF at different ratios as the mobile phases; (D) chiral analyte l on CSP1 with n-hex:IPA:CHCl3 at different ratios as the mobile phases
The addition of acidic and basic modifiers to the mobile phase (FA or DEA, each 1.0 vol%) slightly changed the separation performance. With the two new mobile phases (n-hex:IPA:FA, 90:10:1, v/v/v; and n-hex:IPA:DEA, 90:10:1, v/v/v), all of the selected chiral analytes were partially separated on CSP1 except j and l, while the two analytes d and f could now be partially separated on CSP2. The enantioseparation performance of CSP1 was generally higher than the one of CSP2. The reasons for this are not yet clear. Different geometries and spatial alignments of the CS during the coating and immobilization process could lead to those differences in the enantioseparation performance between the two CSPs.
Further studies with smaller silica particles and higher surface areas, allowing higher efficiencies and consequently higher selector loadings with higher retention and selectivity, are ongoing. A previous comparison from our work (Bui et al. 2022b) with coated materials carrying 20 wt% of selector has shown that the overall performance matched that of commercial reference materials. However, with the present silica dimensions (i.e., relatively low surface area), it was not possible to achieve such high selector loadings (vide supra). It was all the more surprising that a generally higher enantioseparation performance was observed in the case of the immobilized-type CSP versus the coated-type one, which is quite an encouragement for further studies of this immobilization method.
Two categories of “non-standard” mobile phases—with strong eluents, such as THF and CHCl3—at different ratios were selected to study the influence of eluent modification on the enantioseparation performance as well as to evaluate the stability of CSP1. We intended to demonstrate that it is possible to run the chromatographic system also with mobile phases that would dissolve the CS in coated-only column fillings. This applies especially to THF, which was used for dissolution and immobilization during material preparation. The respective chromatographic data are summarized in Tables S4–5. Representative HPLC chromatograms of chiral analyte i using n-hex:IPA:THF (at different ratios) and chiral analyte l using n-hex:IPA:CHCl3 (at different ratios) as the mobile phases are shown in Fig. 6C, D. In Fig. 7, the respective Rs values on CSP1 are displayed.
Increasing the percentage of THF in the mobile phase generally reduced the Rs of the chiral analytes on CSP1. The significant influence of THF can be nicely illustrated by means of chiral analyte d. It was baseline separated on CSP1 with n-hex:IPA (90:10, v/v, Rs = 1.68), but only partially separated at higher THF contents (Rs = 1.33, 1.43, and 0.92, corresponding to 1, 3, and 5% of THF in the mobile phase, see also Table S4). The effect of CHCl3 on the separation of the selected chiral analytes was not always following such a general trend. Compound d was baseline separated on CSP1 using the “standard” eluent n-hex:IPA (90:10, v/v, Rs = 1.68). The resolution increased to 1.96 when 1% of CHCl3 was added, but decreased again when the percentage of CHCl3 was further increased (see Fig. 7B and Table S5). The chiral analyte l could not be separated on CSP1 with n-hex:IPA (90:10, v/v) and n-hex:IPA:CHCl3 (89:10:1, v/v/). However, when 3 or 5% of CHCl3 were added, the selectivity increased and the compound was partially separated (Rs = 0.45 and 0.41, respectively).
Conclusions and outlook
Aminolysis of cellulose carbonates and isocyanate chemistry were optimized, and a three-step, one-pot procedure was employed to synthesize a cellulose 3,5-dimethylphenyl carbamate-type chiral selector carrying propynyl carbamate functional groups (DS approx. 0.14). The compounds' structures were confirmed by both FTIR spectroscopy, solid-state, and solution-state NMR spectroscopy. Additionally, the chiral selector was characterized by GPC and elemental analysis. In parallel, the synthesis of a modified silica gel carrier (3-azidopropyl-functionalized silica gel) via a 3-chloropropyl intermediate was carried out. The pre-functionalized silica gel matrices were characterized by both solid-state 29Si and 13C NMR as well as EA.
The CS carrying alkyne moieties was immobilized on 3-azidopropyl-functionalized silica gel by Cu(I)-catalyzed alkyne-azide cycloaddition click chemistry, in which a selector loading of 6 wt% was achieved. Attempts to increase the loading amount by increasing the number of anchor points (alkyne-groups) on the CS and azido-groups on silica gel were made but were unsuccessful given the low accessible surface area of the silica material. With different silica particle characteristics (especially silica materials with higher surface area), loading amounts comparable to commercially used 20 wt% can be achieved also with this mild immobilization protocol as we will demonstrate in follow-up communications.
The enantioseparation performance of the immobilized CSP was evaluated by using twelve chiral analytes and different eluents, and was compared to a coated-type CSP of the same silica material, selector quality and quantity. The immobilized CSP generally performed better than its coated counterpart. The reasons for this—somewhat unexpected but satisfying—finding need to be further studied in more detail, especially by using silica particles with higher surface area and a selector loading in the range of 20 wt%.
In this contribution, we present a new strategy for the synthesis of chiral selectors by a combination of oxycarbonylation/aminolysis and isocyanate chemistry, as well as the stable and robust immobilization of respective alkyne-carrying carbamate-type selectors onto silica by a mild click chemical protocol and demonstrate the promising performance characteristics of this immobilized CSP in enantioseparation.
References
Aflak N, Ben El Ayouchia H, Bahsis L, El M, El M, Julve M, Rafqah S, Anane H, Stiriba SE (2019) Sustainable construction of heterocyclic 1,2,3-triazoles by strict click [3+2] cycloaddition reactions between azides and alkynes on copper/carbon in water. Front Chem 7:81. https://doi.org/10.3389/fchem.2019.00081
Ahuja S (1997) Chiral separations and technology: an overview. In: Ahuja S (ed) Chiral separations: applications and technology. Amer Chemical Society, Washington DC. https://doi.org/10.1080/10826079808005871
Alvarez-Rivera G, Bueno M, Ballesteros-Vivas D, Cifuentes A (2020) Chiral analysis in food science. Trends Anal Chem 123:115761. https://doi.org/10.1016/j.trac.2019.115761
Bae IA, Park JH, Choi SH (2011) Synthesis of chiral stationary phase via surface-initiated atom transfer radical polymerization of vinylated cellulose 3,5-dimethylphenylcarbamate. Polym Int 60(5):833–838. https://doi.org/10.1002/pi.3027
Bui CV, Rosenau T, Hettegger H (2021) Polysaccharide-and β-Cyclodextrin-based chiral selectors for enantiomer resolution: recent developments and applications. Molecules 26(14):4322. https://doi.org/10.3390/molecules26144322
Bui CV, Rosenau T, Hettegger H (2022a) Synthesis of polyanionic cellulose carbamates by homogeneous aminolysis in an ionic liquid/DMF medium. Molecules 27(4):1384. https://doi.org/10.3390/molecules27041384
Bui CV, Rosenau T, Hettegger H (2022b) Synthesis by carbonate aminolysis and chiral recognition ability of cellulose 2,3-bis(3,5-dimethylphenyl carbamate)-6-(α-phenylethyl carbamate) selectors. Cellulose (2022). https://doi.org/10.1007/s10570-022-04898-8
Burke AJ, Marques CS (2015) Catalytic arylation methods: from the academic lab to industrial processes. Wiley, Weinheim. https://doi.org/10.1002/anie.201509471
Carrão DB, Perovani IS, de Albuquerque NCP, de Oliveira ARM (2019) Enantioseparation of pesticides: A critical review. Trends Analyt Chem 122:115719. https://doi.org/10.1016/j.trac.2019.115719
Chang L, Zhang J, Chen W, Zhang M, Yin C, Tian W, Luo Z, Liu W, He J, Zhang J (2018) Controllable synthesis of cellulose benzoates for understanding of chiral recognition mechanism and fabrication of highly efficient chiral stationary phases. Anal Methods 10(24):2844–2853. https://doi.org/10.1039/C8AY00162F
Chankvetadze B (2019) Polysaccharide-based chiral stationary phases for enantioseparations by high-performance liquid chromatography: an overview. In: Scriba GKE (ed) Chiral separations: methods and protocols. Humana Press, Totowa. https://doi.org/10.1007/s00216-019-02144-y
Chankvetadze B, Ikai T, Yamamoto C, Okamoto Y (2004) High-performance liquid chromatographic enantioseparations on monolithic silica columns containing a covalently attached 3,5-dimethylphenylcarbamate derivative of cellulose. J Chromatogr A 1042(1–2):55–60. https://doi.org/10.1016/j.chroma.2004.05.011
Chebrouk F, Madani K, Cherfaoui B, Boukenna L, Válega M, Mendes RF, Paz FAA, Bachari K, Talhi O, Silva AMS (2019) Hemi-synthesis of chiral imine, benzimidazole and benzodiazepines from essential oil of Ammodaucus leucotrichus subsp. leucotrichus. Molecules 24(5):975. https://doi.org/10.3390/molecules24050975
Chen JL, Hsieh KH (2011) Nanochitosan crosslinked with polyacrylamide as the chiral stationary phase for open-tubular capillary electrochromatography. Electrophoresis 32(3–4):398–407. https://doi.org/10.1002/elps.201000410
Chen X, Zou H, Zhang Q, Ni J, Zhang Z (2002) Synthesis of chemically bonded cellulose trisphenylcarbamate chiral stationary phases for enantiomeric separation. J Chromatogr Sci 40(6):315–320. https://doi.org/10.1093/chromsci/40.6.315
Chen X, Zou H, Ni J, Feng S (2003a) Synthesis and characteristics of composite chiral stationary phases based on cellulose derivatives. J Sep Sci 26(1–2):29–36. https://doi.org/10.1002/jssc.200390010
Chen X, Liu Y, Qin F, Kong L, Zou H (2003b) Synthesis of covalently bonded cellulose derivative chiral stationary phases with a bifunctional reagent of 3-(triethoxysilyl) propyl isocyanate. J Chromatogr A 1010(2):185–194. https://doi.org/10.1016/s0021-9673(03)01104-x
Chen X, Jin W, Qin F, Liu Y, Zou H, Guo B (2003c) Capillary electrochromatographic separation of enantiomers on chemically bonded type of cellulose derivative chiral stationary phases with a positively charged spacer. Electrophoresis 24(15):2559–2566. https://doi.org/10.1002/elps.200305484
Chen XM, Yamamoto C, Okamoto Y (2006) One-pot synthesis of polysaccharide 3,5-dimethylphenylcarbamates having a random vinyl group for immobilization on silica gel as chiral stationary phases. J Sep Sci 29(10):1432–1439. https://doi.org/10.1002/jssc.200600006
D’Orazio G (2020) Chiral analysis by nano-liquid chromatography. Trends Analyt Chem 125:115832. https://doi.org/10.1016/j.trac.2020.115832
Davankov VA (1997) Analytical chiral separation methods. Pure Appl Chem 69(7):1469–1474. https://doi.org/10.1351/pac199769071469
Dong X, Wu R, Dong J, Wu M, Zhu Y, Zou H (2008) The covalently bonded cellulose tris(3,5-dimethylphenylcarbamate) on a silica monolithic capillary column for enantioseparation in capillary electrochromatography. J Chromatogr B 875(1):317–322. https://doi.org/10.1016/j.jchromb.2008.05.019
Elschner T, Ganske K, Heinze T (2013) Synthesis and aminolysis of polysaccharide carbonates. Cellulose 20(1):339–353. https://doi.org/10.1007/s10570-012-9819-9
Elschner T, Kötteritzsch M, Heinze T (2014) Synthesis of cellulose tricarbonates in 1-butyl-3-methylimidazolium chloride/pyridine. Macromol Biosci 14(2):161–165. https://doi.org/10.1002/mabi.201300345
Fanali C, D’Orazio G, Gentili A, Fanali S (2019) Analysis of enantiomers in products of food interest. Molecules 24(6):1119. https://doi.org/10.3390/molecules24061119
Fernandes C, Teixeira J, Pinto MMM, Tiritan ME (2021) Strategies for preparation of chiral stationary phases: progress on coating and immobilization methods. Molecules 26(18):5477. https://doi.org/10.3390/molecules26185477
Franco P, Senso A, Minguillón C, Oliveros L (1998) 3,5-Dimethylphenylcarbamates of amylose, chitosan and cellulose bonded on silica gel: comparison of their chiral recognition abilities as high-performance liquid chromatography chiral stationary phases. J Chromatogr A 796(2):265–272. https://doi.org/10.1016/s0021-9673(97)01004-2
Francotte ER (2001) Enantioselective chromatography as a powerful alternative for the preparation of drug enantiomers. J Chromatogr A 906(1–2):379–397. https://doi.org/10.1016/s0021-9673(00)00951-1
Ganske K, Heinze T (2018) Evaluation of the synthesis of soluble aromatic cellulose carbonates of low degree of substitution. Macromol Chem Phys 219(15):1800152. https://doi.org/10.1002/macp.201800152
Garcés J, Franco P, Oliveros L, Minguillón C (2003) Mixed cellulose-derived benzoates bonded on allylsilica gel as HPLC chiral stationary phases: Influence of the introduction of an aromatic moiety in the fixation substituent. Tetrahedron Asymmetry 14(9):1179–1185. https://doi.org/10.1016/S0957-4166(03)00176-9
He YW, Dong CZ, Zhao JY, Ma LL, Li YH, Aisa HA (2014) 1,2,3-Triazole-containing derivatives of rupestonic acid: Click-chemical synthesis and antiviral activities against influenza viruses. Eur J Med Chem 76:245–255. https://doi.org/10.1016/j.ejmech.2014.02.029
Henniges U, Kostic M, Borgards A, Rosenau T, Potthast A (2011) Dissolution behavior of different celluloses. Biomacromol 12(4):871–879. https://doi.org/10.1021/bm101555q
Hettegger H, Kohout M, Mimini V, Lindner W (2014) Novel carbamoyl type quinine and quinidine based chiral anion exchangers implementing alkyne–azide cycloaddition immobilization chemistry. J Chromatogr A 1337:85–94. https://doi.org/10.1016/j.chroma.2014.02.026
Hettegger H, Sumerskii I, Sortino S, Potthast A, Rosenau T (2015a) Silane meets click chemistry: towards the functionalization of wet bacterial cellulose sheets. Chemsuschem 8(4):680–687. https://doi.org/10.1002/cssc.201402991
Hettegger H, Gorfer M, Sortino S, Fraix A, Bandian D, Rohrer C, Harreither W, Potthast A, Rosenau T (2015b) Synthesis, characterization, and photo-bactericidal activity of silanized xanthene-modified bacterial cellulose membranes. Cellulose 22(5):3291–3304. https://doi.org/10.1007/s10570-015-0715-y
Hettegger H, Beaumont M, Potthast A, Rosenau T (2016) Aqueous modification of nano-and microfibrillar cellulose with a Click synthon. Chemsuschem 9(1):75–79. https://doi.org/10.1002/cssc.201501358
Huang G, Ou J, Zhang X, Ji Y, Peng X, Zou H (2014) Synthesis of novel perphenylcarbamated β-cyclodextrin based chiral stationary phases via thiol-ene click chemistry. Electrophoresis 35(19):2752–2758. https://doi.org/10.1002/elps.201400248
Ikai T, Okamoto Y (2009) Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography. Chem Rev 109(11):6077–6101. https://doi.org/10.1021/cr8005558
Ikai T, Yamamoto C, Kamigaito M, Okamoto Y (2006) Efficient immobilization of cellulose phenylcarbamate bearing alkoxysilyl group onto silica gel by intermolecular polycondensation and its chiral recognition. Chem Lett 35(11):1250–1251. https://doi.org/10.1246/cl.2006.1250
Ikai T, Yamamoto C, Kamigaito M, Okamoto Y (2007a) Immobilization of polysaccharide derivatives onto silica gel: facile synthesis of chiral packing materials by means of intermolecular polycondensation of triethoxysilyl groups. J Chromatogr A 1157(1–2):151–158. https://doi.org/10.1016/j.chroma.2007.04.054
Ikai T, Yamamoto C, Kamigaito M, Okamoto Y (2007b) Immobilized polysaccharide derivatives: chiral packing materials for efficient HPLC resolution. Chem Rec 7(2):91–103. https://doi.org/10.1002/tcr.20107
Jin L, Gao W, Li L, Ye J, Lin C, Liu W (2010) Enantioseparation and enantioselective analysis of chiral herbicides. In: Soloneski S, Laramendy M (eds) Herbicides, theory applications. IntechOpen, Rijeka. https://doi.org/10.5772/1430
Jusner P, Bausch F, Schiehser S, Schwaiger E, Potthast A, Rosenau T (2022) Protocol for characterizing the molar mass distribution and oxidized functionality profiles of aged transformer papers by gel permeation chromatography (GPC). Cellulose 29(4):2241–2256
Kacprzak KM, Lindner W (2011) Novel Pirkle-type quinine 3,5-dinitrophenylcarbamate chiral stationary phase implementing click chemistry. J Sep Sci 34(18):2391–2396. https://doi.org/10.1002/jssc.201100395
Kacprzak KM, Maier NM, Lindner W (2006) Highly efficient immobilization of Cinchona alkaloid derivatives to silica gel via click chemistry. Tetrahedron Lett 47(49):8721–8726. https://doi.org/10.1016/j.tetlet.2006.10.018
Kacprzak KM, Maier NM, Lindner W (2011) Triazolo-linked cinchona alkaloid carbamate anion exchange-type chiral stationary phases: synthesis by click chemistry and evaluation. J Chromatogr A 1218(11):1452–1460. https://doi.org/10.1016/j.chroma.2011.01.031
Kimata K, Tsuboi R, Hosoya K, Tanaka N (1993) Chemically bonded chiral stationary phase prepared by the polymerization of cellulose p-vinylbenzoate. Anal Methods Instrum 1:23–29
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021. https://doi.org/10.1002/1521-3773(20010601)40:11%3c2004::AID-ANIE2004%3e3.0.CO;2-5
Kubota T, Yamamoto C, Okamoto Y (2004) Phenylcarbamate derivatives of cellulose and amylose immobilized onto silica gel as chiral stationary phases for high-performance liquid chromatography. J Polym Sci A Polym Chem 42(18):4704–4710. https://doi.org/10.1002/pola.20372
Lämmerhofer M (2010) Chiral recognition by enantioselective liquid chromatography: mechanisms and modern chiral stationary phases. J Chromatogr A 1217(6):814–856. https://doi.org/10.1016/j.chroma.2009.10.022
Lao W, Gan J (2006) High-performance liquid chromatographic separation of imidazolinone herbicide enantiomers and their methyl derivatives on polysaccharide-coated chiral stationary phases. J Chromatogr A 1117(2):184–193. https://doi.org/10.1016/j.chroma.2006.03.094
Lauria A, Delisi R, Mingoia F, Terenzi A, Martorana A, Barone G (2014) Almerico AM (2014) 1,2,3-Triazole in heterocyclic compounds, endowed with biological activity, through 1,3-dipolar cycloadditions. Eur J Org Chem 16:3289–3306. https://doi.org/10.1002/ejoc.201301695
Li L, Wang H, Shuang Y, Li L (2019) The preparation of a new 3,5-dichlorophenylcarbamated cellulose-bonded stationary phase and its application for the enantioseparation and determination of chiral fungicides by LC-MS/MS. Talanta 202:494–506. https://doi.org/10.1016/j.talanta.2019.05.011
Li L, Yuan XT, Shi ZG, Xu LY (2020) Chiral stationary phase based on cellulose derivative coated polymer microspheres and its separation performance. J Chromatogr A 1623:461154. https://doi.org/10.1016/j.chroma.2020.461154
Li R, Wang Z, Zhang Y, Chen Z, Sang N, Wang M, Shi H (2022) A novel enantioseparation method and enantioselective dissipation of novaluron in tomatoes using ultrahigh-performance liquid chromatography tandem mass spectrometry via a box-Behnken design. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.2c02166
Liebert T, Hänsch C, Heinze T (2006) Click chemistry with polysaccharides. Macromol Rapid Commun 27(3):208–213. https://doi.org/10.1002/marc.200500686
Lin C, Fan J, Liu W, Chen X, Ruan L, Zhang W (2018) A new single-urea-bound 3,5-dimethylphenylcarbamoylated β-cyclodextrin chiral stationary phase and its enhanced separation performance in normal-phase liquid chromatography. Electrophoresis 39(2):348–355. https://doi.org/10.1002/elps.201700273
Liu RQ, Bai LY, Zhang YJ, Zhang YP (2013) Green synthesis of a typical chiral stationary phase of cellulose tris(3,5-dimethylphenylcarbamate). Chem Cent J 7(1):1–5. https://doi.org/10.1186/1752-153X-7-129
Lüttringhaus A, Hess U, Rosenbaum HJ (1967) Optisch aktives 4.5.6.7-Dibenzo-1.2-dithiacyclooctadien. Z Naturforsch B 22(12):1296–1300. https://doi.org/10.1515/znb-1967-1212
Martín-Biosca Y, García-Ruiz C, Marina ML (2001) Enantiomeric separation of chiral phenoxy acid herbicides by electrokinetic chromatography. Application to the determination of analyte-selector apparent binding constants for enantiomers. Electrophoresis 22(15):3216–3225. https://doi.org/10.1002/1522-2683(200109)22:15%3c3216::AID-ELPS3216%3e3.0.CO;2-X
Merino MED, Echevarría RN, Lubomirsky E, Padro JM, Castells CB (2019) Enantioseparation of the racemates of a number of pesticides on a silica-based column with immobilized amylose tris(3-chloro-5-methylphenylcarbamate). Microchem J 149:103970. https://doi.org/10.1016/j.microc.2019.103970
Miaomiao H, Xiaosa J, Han Y, Xiao L, Yadong L, Shengxiang J (2017) Controlled synthesis, immobilization and chiral recognition of carboxylic acid functionalized cellulose tris(3,5-dimethylphenylcarbamate). Carbohydr Polym 172:223–229. https://doi.org/10.1016/j.carbpol.2017.05.049
Minguillón C, Franco P, Oliveros L, López P (1996) Bonded cellulose-derived high-performance liquid chromatography chiral stationary phases I: Influence of the degree of fixation on selectivity. J Chromatogr A 728(1–2):407–414. https://doi.org/10.1016/0021-9673(95)01123-4
Mukherjee A, Bera A (2012) Importance of chirality and chiral chromatography in pharmaceutical industry: a detailed study. J Chem Pharm 2(4):334–346
Okamoto Y, Kaida Y (1994) Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J Chromatogr A 666(1–2):403–419. https://doi.org/10.1016/0021-9673(94)80400-1
Okamoto Y, Kawashima M, Hatada K (1984a) Useful chiral packing materials for high-performance liquid chromatographic resolution of enantiomers: phenylcarbamates of polysaccharides coated on silica gel. J Am Chem Soc 106(18):5357–5359. https://doi.org/10.1021/ja00330a057
Okamoto Y, Kawashima M, Yamamoto K, Hatada K (1984b) Useful chiral packing materials for high-performance liquid chromatographic resolution. Cellulose triacetate and tribenzoate coated on macroporous silica gel. Chem Lett 13(5):739–742. https://doi.org/10.1246/cl.1984.739
Okamoto Y, Kawashima M, Hatada K (1986) Chromatographic resolution: XI: Controlled chiral recognition of cellulose triphenylcarbamate derivatives supported on silica gel. J Chromatogr A 363(2):173–186. https://doi.org/10.1016/S0021-9673(01)83736-5
Okamoto Y, Aburatani R, Miura SI, Hatada K (1987) Chiral stationary phases for HPLC: Cellulose tris(3,5-dimethylphenylcarbamate) and tris(3,5-dichlorophenylcarbamate) chemically bonded to silica gel. J Liq Chromatogr 10(8–9):1613–1628. https://doi.org/10.1080/01483918708066791
Onishi T, Ueda T, Yoshida K, Uosaki K, Ando H, Hamasaki R, Ohnishi A (2022) Characteristic and complementary chiral recognition ability of four recently developed immobilized chiral stationary phases based on amylose and cellulose phenyl carbamates and benzoates. Chirality. https://doi.org/10.1002/chir.23446
Padró JM, Keunchkarian S (2018) State-of-the-art and recent developments of immobilized polysaccharide-based chiral stationary phases for enantioseparations by high-performance liquid chromatography (2013–2017). Microchem J 140:142–157. https://doi.org/10.1016/j.microc.2018.04.017
Pedersen DS (2011) Abell A (2011) 1,2,3-Triazoles in peptidomimetic chemistry. Eur J Org Chem 13:2399–2411. https://doi.org/10.1002/ejoc.201100157
Peng GM, Wu SQ, Fang ZL, Zhang WG, Bin Zhang Z, Fan J, Zheng SR, Wu SS, Ng SC (2012) Preparation and chiral separation of a novel immobilized cellulose-based chiral stationary phase in high-performance liquid chromatography. J Chromatogr Sci 50(6):516–522. https://doi.org/10.1093/chromsci/bms047
Pingaew R, Saekee A, Mandi P, Nantasenamat C, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2014) Synthesis, biological evaluation, and molecular docking of novel chalcone–coumarin hybrids as anticancer and antimalarial agents. Eur J Med Chem 85:65–76. https://doi.org/10.1016/j.ejmech.2014.07.087
Potthast A, Rosenau T, Sartori J, Sixta H, Kosma P (2003) Hydrolytic processes and condensation reactions in the cellulose solvent system N, N-dimethylacetamide/lithium chloride: Part 2: degradation of cellulose. Polymer 44(1):7–17. https://doi.org/10.1016/S0032-3861(02)00751-6
Pourjavadi A, Seidi F, Afjeh SS, Nikoseresht N, Salimi H, Nemati N (2011) Synthesis of soluble N-functionalized polysaccharide derivatives using phenyl carbonate precursor and their application as catalysts. Starch-Stärke 63(12):780–791. https://doi.org/10.1002/star.201100064
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB (2002) A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 114(14):2708–2711. https://doi.org/10.1002/1521-3757(20020715)114:14%3c2708::AID-ANGE2708%3e3.0.CO;2-0
Ruiz-Rodríguez L, Aguilar A, Díaz AN, Sánchez FG (2015) Enantioseparation of the fungicide imazalil in orange juice by chiral HPLC. Study on degradation rates and extractive/enrichment techniques. Food Chem 178:179–185. https://doi.org/10.1016/j.foodchem.2015.01.004
Salon MCB, Gerbaud G, Abdelmouleh M, Bruzzese C, Boufi S, Belgacem MN (2007) Studies of interactions between silane coupling agents and cellulose fibers with liquid and solid-state NMR. Magn Reson Chem 45(6):473–483. https://doi.org/10.1002/mrc.1994
Shen J, Okamoto Y (2016) Efficient separation of enantiomers using stereoregular chiral polymers. Chem Rev 116(3):1094–1138. https://doi.org/10.1021/acs.chemrev.5b00317
Shen J, Liu S, Li P, Shen X, Okamoto Y (2012) Controlled synthesis and chiral recognition of immobilized cellulose and amylose tris(cyclohexylcarbamate)s/3-(triethoxysilyl) propylcarbamates as chiral packing materials for high-performance liquid chromatography. J Chromatogr A 1246:137–144. https://doi.org/10.1016/j.chroma.2012.05.002
Shi G, Dai X, Zhou Y, Zhang J, Shen J, Wan X (2020) Synthesis and enantioseparation of proline-derived helical polyacetylenes as chiral stationary phases for HPLC. Polym Chem 11(18):3179–3187. https://doi.org/10.1039/D0PY00205D
Silva M, Pérez-Quintanilla D, Morante-Zarcero S, Sierra I, Marina ML, Aturki Z, Fanali S (2017) Ordered mesoporous silica functionalized with β-cyclodextrin derivative for stereoisomer separation of flavanones and flavanone glycosides by nano-liquid chromatography and capillary electrochromatography. J Chromatogr A 1490:166–176. https://doi.org/10.1016/j.chroma.2017.02.012
Singh M, Sethi S, Bhushan R (2020) Liquid chromatographic methods for separation, determination, and bioassay of enantiomers of etodolac: a review. J Sep Sci 43(1):18–30. https://doi.org/10.1002/jssc.201900649
Sletten EM, Bertozzi CR (2011) From mechanism to mouse: a tale of two bioorthogonal reactions. Acc Chem Res 44(9):666–676. https://doi.org/10.1021/ar200148z
Solum EJ, Vik A, Hansen TV (2014) Synthesis, cytotoxic effects, and tubulin polymerization inhibition of 1,4-disubstituted 1,2,3-triazole analogs of 2-methoxyestradiol. Steroids 87:46–53. https://doi.org/10.1016/j.steroids.2014.05.020
Subramanian G (2008) Chiral separation techniques: a practical approach. Wiley, Weinheim. https://doi.org/10.1002/9783527611737
Tan Y, Fan J, Lin C, Tu H, Zheng S, Zhang W (2014) Synthesis and enantioseparation behaviors of novel immobilized 3,5-dimethylphenylcarbamoylated polysaccharide chiral stationary phases. J Sep Sci 37(5):488–494. https://doi.org/10.1002/jssc.201301146
Tang S, Ikai T, Tsuji M, Okamoto Y (2010a) Immobilization of 3,5-dimethylphenylcarbamates of cellulose and amylose onto silica gel using (3-glycidoxypropyl) triethoxysilane as linker. J Sep Sci 33(9):1255–1263. https://doi.org/10.1002/jssc.200900711
Tang S, Ikai T, Tsuji M, Okamoto Y (2010b) Immobilization and chiral recognition of 3,5-dimethylphenylcarbamates of cellulose and amylose bearing 4-(trimethoxysilyl) phenylcarbamate groups. Chirality 22(1):165–172. https://doi.org/10.1002/chir.20722
Tang S, Liu G, Li X, Jin Z, Wang F, Pan F, Okamoto Y (2011) Improved preparation of chiral stationary phases via immobilization of polysaccharide derivative-based selectors using diisocyanates. J Sep Sci 34(15):1763–1771. https://doi.org/10.1002/jssc.201100260
Totobenazara J, Burke AJ (2015) New click chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett 56(22):2853–2859. https://doi.org/10.1016/j.tetlet.2015.03.136
Wang X, Li H, Quan K, Zhao L, Qiu H, Li Z (2021a) Preparation and applications of cellulose-functionalized chiral stationary phases: A review. Talanta 225:121987. https://doi.org/10.1016/j.talanta.2020.121987
Wang Z, Liu S, Zhao X, Tian B, Sun X, Zhang J, Gao Y, Shi H, Wang M (2021b) Enantioseparation and stereoselective dissipation of the novel chiral fungicide pydiflumetofen by ultra-high-performance liquid chromatography tandem mass spectrometry. Ecotoxicol Environ Saf 207:111221. https://doi.org/10.1016/j.ecoenv.2020.111221
Wei Q, Su H, Gao D, Wang S (2019) HPLC with cellulose tris(3,5-dimethylphenyl carbamate) chiral stationary phase: Influence of coating times and coating amount on chiral discrimination. Chirality 31(3):164–173. https://doi.org/10.1002/chir.23044
Yaling W, Alex C (2013) Crystallization based separation of enantiomers. In: Andrushko V, Andrushko N (eds) Stereoselective synthesis of drugs and natural products. Wiley, Karlsruhe. https://doi.org/10.1002/9781118596784
Yamamoto C, Okamoto Y (2004) Optically active polymers for chiral separation. Bull Chem Soc Jpn 77(2):227–257. https://doi.org/10.1246/bcsj.77.227
Yao X, Zheng H, Zhang Y, Ma X, Xiao Y, Wang Y (2016) Engineering thiol-ene click chemistry for the fabrication of novel structurally well-defined multifunctional cyclodextrin separation materials for enhanced enantioseparation. Anal Chem 88(9):4955–4964. https://doi.org/10.1021/acs.analchem.6b00897
Yashima E, Yamamoto C, Okamoto Y (1998) Polysaccharide-based chiral LC columns. Synlett 1998(04):344–360. https://doi.org/10.1055/s-1998-1675
Yin C, Chen W, Zhang J, Zhang M, Zhang J (2019) A facile and efficient method to fabricate high-resolution immobilized cellulose-based chiral stationary phases via thiol-ene click chemistry. Sep Purif Technol 210:175–181. https://doi.org/10.1016/j.seppur.2018.08.002
Ying Z, Ling L, Kunde L, Xinping Z, Weiping L (2009) Enantiomer separation of triazole fungicides by high-performance liquid chromatography. Chirality 21(4):421–427. https://doi.org/10.1002/chir.20607
Yu X, Wang Y, Yang Q, Zhang Z, Ren Q, Bao Z, Yang Y (2020) De novo synthesis of microspheical cellulose 3,5-dichlorophenylcarbamates: an organic-inorganic hybrid chiral stationary phase for enantiospearation. Sep Purif Technol 238:116480. https://doi.org/10.1016/j.seppur.2019.116480
Zhang S, Ong TT, Ng SC, Chan HSO (2007) Chemical immobilization of azido cellulose phenylcarbamate onto silica gel via Staudinger reaction and its application as a chiral stationary phase for HPLC. Tetrahedron Lett 48(31):5487–5490. https://doi.org/10.1016/j.tetlet.2007.05.167
Zhang Q, Shi H, Gao B, Tian M, Hua X, Wang M (2016) Enantioseparation and determination of the chiral phenylpyrazole insecticide ethiprole in agricultural and environmental samples and its enantioselective degradation in soil. Sci Total Environ 542:845–853. https://doi.org/10.1016/j.scitotenv.2015.10.132
Zhao P, Dong X, Chen X, Guo X, Zhao L (2019) Stereoselective analysis of chiral pyrethroid insecticides tetramethrin and α-cypermethrin in fruits, vegetables, and cereals. J Agric Food Chem 67(33):9362–9370. https://doi.org/10.1021/acs.jafc.9b01850
Zhou Y, Liang Q, Zhang Z, Wang Z, Huang M (2020) Chiral separations with crosslinked cellulose derivatives attached onto hybrid silica monolith particles via the thiol-ene click reaction. Anal Methods 12(21):2727–2734. https://doi.org/10.1039/D0AY00772B
Acknowledgments
Peter Frühauf (University of Vienna) is gratefully acknowledged for his support with column packing.
Funding
Open access funding provided by the University of Natural Resources and Life Sciences, Vienna (BOKU). The authors would like to thank the University of Natural Resources and Life Sciences, Vienna (BOKU) and the County of Lower Austria for the financial support in the framework of the “Austrian Biorefinery Center Tulln” (ABCT) and the BOKU doctoral school “Advanced Biorefineries: Chemistry & Materials” (ABC&M). C.V.B. is grateful for an Ernst Mach Grant (ASEA-UNINET, ICM-2019-13801). The financial support by the Gesellschaft für Forschungsförderung Niederösterreich m.b.H. (H.H., project LSC20-002) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
C.V.B, T.R. and H.H. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by C.V.B. The original draft of the manuscript was written by C.V.B., including visualization. Review & editing by T.R. and H.H. Supervision, project administration and funding acquisition by T.R. and H.H. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent for publication
All authors agreed to the publication in the submitted form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bui, C.V., Rosenau, T. & Hettegger, H. Immobilization of a cellulose carbamate-type chiral selector onto silica gel by alkyne-azide click chemistry for the preparation of chiral stationary chromatography phases. Cellulose 30, 915–932 (2023). https://doi.org/10.1007/s10570-022-04932-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04932-9