Abstract
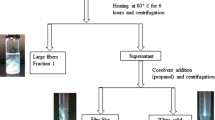
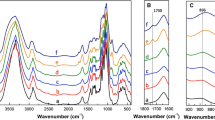
Periodate oxidation breaks the C2–C3 bond in the glucose repeat units of cellulose, forming two vicinal aldehyde groups. When the cellulose is partially oxidized, three products were generated after periodate oxidation: fibrous cellulose, sterically stabilized nanocrystalline cellulose (SNCC) and dialdehyde modified cellulose. Thus, by periodate oxidation alone, we can produce nanocellulose. SNCCs were produced after 26, 42 and 84 h periodate oxidation. Their morphologies were examined by transmission electron microscopy, which show that the three SNCCs have similar diameters (5–10 nm). In contrast, the average length of SNCC decreases with aldehyde content: from approximately 590 nm after 26 h of oxidation to 100 nm for an oxidation period of 84 h. It indicates that the morphology of SNCC can be well controlled by the degree of periodate oxidation, which depends on the amount of periodate and the reaction time. Equivalent spherical diameters of SNCCs were also examined by dynamic light scattering, and the results correspond closely to the ones observed by TEM. The viscosities of SNCCs were measured by an Ubbelohde viscometer and compared with theory. Because the length of SNCC particles gradually reduces while their diameters remain almost the same, we propose that periodate reacts preferentially with the amorphous region of cellulose. After most of the amorphous regions have reacted, the reaction proceeds at the boundary of amorphous and crystalline regions, creating a reaction front that advances towards the crystalline regions, thus continually shortening them. Dynamic light scattering experiments on SNCC suspensions when adding cosolvents into them proved that SNCCs were sterically stabilized in water.






Similar content being viewed by others
References
Dash R, Elder T, Ragauskas AJ (2012) Grafting of model primary amine compounds to cellulose nanowhiskers through periodate oxidation. Cellulose 19:2069–2079
Dhont JKG, Briels WJ (2003) Viscoelasticity of suspensions of long, rigid rods. Colloids Surf A 213:131–156
Dong XM, Kimura T, Revol JF, Gray DG (1996) Effects of ionic strength on the isotropic–chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 12:2076–2082
Gal’braikh LS, Rogovin ZA (1971) Cellulose and cellulose derivatives. In: Bikales NM, Segal L (eds) New York, p 893
Gurvich AE, Lechtzind EV (1982) High capacity immunoadsorbents based on preparations of reprecipitated cellulose. Mol Immunol 19(4):637–640
Jowkarderis L, van de Ven TGM (2014) Intrinsic viscosity of aqueous suspensions of cellulose nanofibrils. Cellulose 21:2511–2517
Kim JU, Kua S (2001) Ion-exchange chromatography by dicarboxyl cellulose gel. J Chromatogr 29:919
Kim UJ, Wada M, Kuga S, Okano T, Kondo T (2000) Periodate oxidation of crystalline cellulose. Biomacromolecules 1:488
Liu X, Wang L, Song X, Song H, Zhao J, Wang S (2012) A kinetic model for oxidative degradation of bagasse pulp fiber by sodium periodate. Carbohydr Polym 90:218–223
Mansfield M, Douglas J (2008) Transport properties of rodlike particle. Macromolecules 41:5422–5432
Mikhail SZ, Kimel WR (1963) Densities and viscosities of 1-propanol-water mixtures. J Chem Eng Data 8:3
Napper DH (1984) Polymeric stabilization of colloidal dispersions. Chapter 5, Academic Press, London
Pääkkö M, Ankerfors M, Kosonen H, Nykänen A, Ahola S, Österberg M, Ruokolainen J, Laine J, Larsson PT, Ikkala O, Lindström T (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong Gels. Biomacromolecules 8(6):1934–1941
Rinaudo M (2010) Periodate oxidation of methylcellulose: characterization and properties of oxidized derivatives. Polymers 2:505–521
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 10:1992–1996
Sassi J, Chanzy H (1995) Ultrastructural aspects of the acetylation of cellulose. Cellulose 2:111–127
Sirviö JA, Hasa T, Ahola J, Liimatainen H, Niinimäki J, Hormi O (2015) Phosphonated nanocelluloses from sequential oxidative-reductive treatment-physicochemical characteristics and thermal properties. Carbohydr Polym 133:524–532
Sun B, Hou Q, Liu Z, Ni Y (2015) Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 22:1135–1146
van de Ven TGM (1989) Colloidal hydrodynamics. Academic Press Limited, London
van de Ven TGM, Tejado A, Alam MN, Antal M (2012) Salt-induced acceleration of chemical reactions in cellulose nanopores. Cellulose 19:517–522
Wågberg L, Decher G, Norgren M, Lindström T, Ankerfors M, Axnäs K (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24(3):784–795
Yang H (2011) Investigation and characterization of oxidized cellulose and cellulose nanofiber films. MSc thesis of department of Chemistry, McGill
Yang H, Tejado A, Alam MN, Antal M, van de Ven TGM (2012) Films prepared from electrosterically stabilized nanocrystalline cellulose. Langmuir 28:7834–7842
Yang H, Chen D, van de Ven TGM (2015) Preparation and characterization of sterically stabilized nanocrystalline cellulose obtained by periodate oxidation of cellulose fibres. Cellulose 22:1743–1752
Ye J, Xiong J, Liang W (1998) Effect of the pH on the synthesis of o-phenylenediimido cellulose and its fluorescence behavior. J Funct Polymers 11:539–543
Acknowledgments
The authors would like to acknowledge NSERC for financial support through the Green FIBRE strategic research network, FPInnovations and NSERC for the funding of an Industrial Research Chair, and Domtar lnc. for donating pulp samples. They also would like to thank Dr. Md. Nur Alam and Han Yang for providing recipes of periodate oxidation, and Dr. Georgios Rizis for training in the use of dynamic light scattering.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., van de Ven, T.G.M. Morphological changes of sterically stabilized nanocrystalline cellulose after periodate oxidation. Cellulose 23, 1051–1059 (2016). https://doi.org/10.1007/s10570-016-0862-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-016-0862-9