Abstract
Sugar maple (Acer saccharum, Mill.) wood samples were exposed to the flowing afterglow of a N2 dielectric barrier discharge (DBD) open to ambient air. Freshly-sanded wood surfaces were hydrophilized by the treatment. The dynamic behaviour of water droplets on hardwood samples further reveals a volumetric effect of treatment as well as a modification of the topmost surface. Analysis of the discharge properties by optical emission spectroscopy (OES) indicates that the neutral gas temperature (determined from the rovibrational spectrum of N2) was close to room temperature, thus ruling out wood modification due to heat transfer. OES spectra combined with a collisional-radiative model also reveals significant concentration of metastable N2(A) states and UV photons (in particular those from the NOγ system in the 200–300 nm range) in the discharge. To better examine the role of UV irradiation, wood samples were exposed to the late afterglow of a low-pressure N2/O2 plasma optimized for UV emission from the NO systems. Wood hydrophilization was observed only for samples directly exposed to the late afterglow and not for those contained in a UV-transparent enclosure. Wood hydrophilization in the DBD is thus not directly related to UV irradiation; these energetic photons rather participate [along with N2(A) metastables] in the formation of other active species, in particular atomic oxygen and ozone due to the open-air configuration. The role of ozone was confirmed by treatments in an ozone generator, showing dynamic wettability comparable to the ones achieved after treatment in the flowing afterglows of the atmospheric-pressure N2 DBD and low-pressure N2–O2 plasma. FTIR spectra of wood samples treated in the three systems (DBD, N2–O2 plasma, and ozone generator) indicate an increased lignin content due to the possible development of lignin precipitates, corroborating the effect of atomic oxygen and ozone.
Similar content being viewed by others
Introduction
Wood is considered a fine and strong material, but it requires regular and meticulous maintenance. The advantages of wood as a material of construction lie in its versatility, strength-to-weight characteristics, and its sustainability as a green-material. Its bio-polymeric structure, however, renders it susceptible to degradation. Weathering and biological decay, from fungus and mold, are major factors that affect the integrity of wooden structures. The current approach for preventing decay is the application of wet coatings either as paints and stains to the wood surface, or by pressure-treatment to impregnate the wood itself with a protective material. Chemical treatments typically offer an inexpensive environmental barrier, but they can be costly to the environment itself. Run-off from treated wood surfaces, dangerous chemicals released from burning pressure-treated wood, and wasted or improperly disposed chemicals all pose significant threats to the environment. Furthermore, the stability of surface treatments depends greatly on the state of the wood and the location of its use. Exposed wood surfaces often require periodic re-application of chemical barriers, which increases both the costs and the environmental risks of the treatments. Long-term heating (or torrefaction) is another treatment that is commonly used to render wood more workable and less prone to decay. While not requiring chemical treatments, wood treated this way cannot be used in all environments and is more sensitive to moisture damage. Furthermore, the heating itself is a destructive process altering the mechanical properties and limiting the lifetime of the wooden material (Bekhta and Niemz 2003).
Cold plasmas represent a promising development for tailoring the surface properties of many polymeric materials (Egitto and Matienzo 1994; Hegemann et al. 2003; Akishev et al. 2008). Such plasmas offer the additional advantage of operation at near-ambient temperatures, making them ideal for the treatment of thermally sensitive materials. Plasma treatment of wood surfaces has been investigated only recently, with the main interest being to improve adhesion between an applied protection barrier and the wood surface beneath it (Podgorski and Roux 1999; Custódio et al. 2009; Acda et al. 2011). Among cold plasma sources, dielectric barrier discharges (DBDs) have been identified as the most industrially relevant approach for plasma-induced modification of wood. DBD sources are capable of non-equilibrium operation at atmospheric pressure, which is considered to be the best option for research into large-scale treatment for the timber/lumber industry. Non-equilibrium plasma sources operating at low pressure such as those that are used for plasma deposition and etching of thin films in the microelectronic industry, require complex vacuum systems and tightly controlled environments. The batch-wise operation necessitated by vacuum enclosures limits the throughput of treatment processes. This is a serious impediment to the application of low-pressure plasmas in industrial treatment of large volumes of materials with relatively low-added value.
Recently published reports on plasma treatments of wood deal mostly with water contact angle measurements in the interest of decreasing the interfacial tension between wood and water. This is also referred to as hydrophilization. Hydrophilized wood surfaces have been reported after DBD-plasma treatment in N2, O2, air, and noble gases (Mahlberg et al. 1998; Podgorski et al. 2000; Rehn and Viöl 2003; Wolkenhauer et al. 2008; Avramidis et al. 2009; Asandulesa et al. 2010). In some cases, this is accompanied by an oxidation of the surface (Avramidis et al. 2009). That being said, the link between surface wettability and coating adhesion is not yet fully understood (Podgorski et al. 2000; Wolkenhauer et al. 2009). In principle, hydrophilization, resulting in enhanced wettability, should lead to a concurrent enhancement in coating adhesion for water-based protectants. The opposite, however, has also been reported. Plasma treatments that lead to more hydrophobic wood surfaces can also produce better adhesion with a water-based urethane–acrylate coating (Busnel et al. 2010). This is because wettability is not the only factor driving the adhesion properties.
Another approach consists of increasing the hydrophobicity of the wood surface for a better general durability. Native wood surfaces are not hydrophobic, so hydrophobization requires a significant modification of the surface chemistry with non-polar functional groups. Hydrophobic wood surfaces have been reported after treatment in plasmas operated with precursors such as CH4, CF4, C2H2, and C3F6 (Podgorski et al. 2000; Rehn and Viöl 2003; Toriz et al. 2008). In those cases, an increase of the carbon concentration on the surface is observed (Toriz et al. 2008), which indicates that the process involves a plasma-deposited layer. Coating with plasma polymers can also modify the hydrophobicity of wood surfaces. For example, hexamethyldisiloxane (HMDSO) precursors fragment in the plasma to form organosilicon SiOCHx layer on the wood surface (Mahlberg et al. 1998; Denes et al. 1999; Levasseur et al. 2012). Aging studies of such treated wood surfaces have shown improved stability of the water contact angle over a time frame of up to 9 months after treatment (Levasseur et al. 2012).
The study of the plasma-wood interaction is further challenged by the complex chemical and anatomical structure of the material. Wood has numerous components, such as cellulose, hemicellulose, lignin, and weakly bound components called extractives. Studies concerning the modification of ligno-cellulosic materials by plasma treatment show different results depending on the nature of the gas. For example, treatments in Ar and H2 plasmas have been found to reduce hydroxyl functionality in both lignin (Klarhöfer et al. 2010) and cellulose (Klarhöfer et al. 2010; Carlsson and Ström 1991). In O2 containing plasmas, an oxidation of lignin and both an oxidation and a reduction of cellulose are observed (Klarhöfer et al. 2010; Carlsson and Ström 1991).
Considering the complexity of the potential interactions between wood surfaces and DBDs, most authors have only reported parametric studies of the wood modification (for example through changes in the nature of the gas and plasma operating conditions). As a consequence, no details are given concerning the nature and role of plasma-generated species on the wood modification dynamics. In most experimental reports, treatment occurs directly in the discharge with the sample being placed in direct contact with one of the electrodes. Only one experiment has been reported where treatment occurred in the flowing afterglow of a DBD (Busnel et al. 2010). It is well known that sanding of the wood surface removes hydrophobic extractives, which can lead to more hydrophilic surfaces (Aydin 2004). While a comparison between sanding, plasma treatment, and plasma treatment after sanding is available (Wolkenhauer et al. 2009), major changes after plasma treatments of freshly-sanded wood samples have not yet been reported.
In the interest of furthering the understanding of plasma-wood interactions, freshly-sanded hardwood samples (sugar maple) were treated in the flowing afterglow of a N2 DBD open to ambient air. As shown below, N2 gas was chosen for its ability to produce a homogeneous discharge instead of the filamentary discharge usually observed in air DBDs. As for the flowing afterglow configuration, it offers much more versatility than direct plasma treatments. Because wood substrates are placed outside the discharge zone, the plasma properties are much less influenced by the release of wood products. In addition, very large and thick samples can readily be processed, which makes the afterglow configuration more compatible with the industrial needs. We have determined the effect of treatment on hydrophilicity by dynamic water contact angle measurements and have observed the changes in chemical composition of the surface by collecting FTIR and XPS spectra. The aim of this work is to gain insights into the role of the numerous plasma-generated species in the changes observed on the wood surface. A thorough investigation of the plasma characteristics has been followed, focusing on the nature of the active species by optical emission spectroscopy (OES) combined with plasma modeling.
Experimental methods
DBD set-up and plasma diagnostics
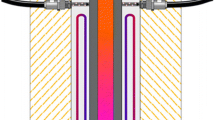
The planar DBD ATMOS setup (Plasmionique, Canada) that was used for treatments of wood samples is described in a diagram in Fig. 1a. The system open to ambient air produces DBD between two quartz-covered circular metallic electrodes, having an area of 285 cm2 and a diameter of 19 cm. The dielectric thickness was 0.05 cm and the inter-electrode gap was 0.14 cm. A variable alternating current generator, driven at 9 kHz, powered the discharge. An amplifier was connected to the generator to provide sufficient power to ignite and sustain the discharge. A water-cooling system was installed to cool the electrodes when higher discharge currents are used. The equivalent circuit for the DBD, in operation, is given in Fig. 1b. It includes the capacitance of both quartz plates (dielectric capacitance), the capacitance of the gas (giving rise to the displacement current), and the resistance of the discharge in operation (giving rise to the Ohmic or discharge current). Current–voltage (I–V) characteristics were measured using a high-voltage probe (for the applied voltage) and a Rogowski coil (for the current). Based on Ref. Naudé et al. (2005), the time evolutions of the discharge voltage (after subtraction of the dielectric voltage from the measured applied voltage) and the discharge current (after subtraction of the displacement current from the measured current) were calculated. As shown in Fig. 1c, the discharge ignites for a voltage close to 3.0 kV, which is comparable to the values expected for breakdown in atmospheric pressure N2 for an inter-electrode gap of 0.14 cm. After breakdown, large and continuous discharge current bands are observed, indicating that the DBD operates in a homogenous regime (Naudé et al. 2005). The product of the discharge voltage and discharge current integrated over one oscillation period yields an average power dissipated in the discharge of 40 W.
a Schematic view of the DBD plasma source open to ambient air; b circuit diagram showing the equivalent circuit of the DBD in operation. Wood samples being placed in the flowing afterglow of the DBD, they do not appear in the equivalent circuit and thus do not influence the discharge properties; c Current–voltage characteristics of the DBD obtained after subtraction of the dielectric voltage from the measured applied voltage (to obtain the discharge voltage) and after subtraction of the displacement current from the measured current (to obtain the discharge current). Details on the analysis of the current–voltage characteristics can be found in Ref. Naudé et al. (2005)
Optical emission spectroscopy (OES) measurements were taken using an optical fiber placed under the discharge in the position of the wood sample (Fig. 1a). A SpectraPro 2300i spectrometer with a 1,200 mm−1 grating and coupled to a charged-coupled device (CCD) camera was used to acquire OES spectra between 200 and 900 nm. For a slit width of 10 μm, the spectral resolution of the system (full width at half maximum) was about 0.3 nm. For experiments requiring a higher spectral resolution, for example to analyze the rovibrational structure of N2 for determination of the rotational temperature (see below), a Triax 550 spectrometer with a 3,600 mm−1 grating and an Andor intensified CCD camera was used. In our conditions, the spectral resolution (full width at half maximum) was about 0.03 nm.
Wood sample preparation and treatment
The wood substrates investigated were taken from sugar maple (Acer saccharum, Mill) lumber. Wood samples of 6.0 cm × 10.0 cm with a thickness of 0.5 cm were prepared from longitudinal sections of a single piece of sapwood lumber. Samples were pre-conditioned in a controlled atmosphere at 20 °C and 65 % RH for 1 week. Immediately prior to plasma treatment, samples were sanded with 150-grit silica sandpaper. Sanding has been shown to remove the wood extractives that migrate to the surface, which is important to avoid a false finding caused by simple “cleaning” of the surface by the plasma (Podgorski et al. 2000; Wolkenhauer et al. 2009).
The substrates were exposed to the flowing afterglow of the N2 (99.998 % purity) DBD, as illustrated in Fig. 1a. This was achieved by placing them onto an automatic conveyer system passing under the discharge at a constant speed of 1.5 cm s−1. The number of passes through the flowing afterglow was used as a parameter to control the plasma-treatment duration. Samples were passed through the plasma afterglow either 3, 5 or 10 times. To ensure that the plasma-generated species reached the surface, the substrates were passed beneath the flow at a distance of 0.3 cm from the outlet of the DBD and high N2 gas flow rates (50 L min−1) were used. The system was built with safety guards that limited access to the area near the entry and exit of the treatment area. For this reason, a significant time (between 1 and 2 min) elapsed between passes, while samples were moving on the conveyer both into and out of the plasma system. The wood samples were thus considered to have sufficient time to relax between treatments.
Analysis of treated wood samples
The hydrophilization of the wood samples was investigated by wettability measurements with a dynamic sessile drop method using a FTA200 contact angle goniometer. Measurements were performed several hours (<24 h) after the treatment. 10 μL water droplets were deposited onto the surface while images were captured at a rate of 60 frames per second. Water contact angles were determined digitally using the FTA200 software package. All measurements presented correspond to an average of ten drops over different areas of the sample, with the error bar corresponding to one standard deviation.
Fourier transform infra-red (FTIR) spectroscopy was performed both by transmission using the KBr pellet technique and by Attenuated Total Reflectance (ATR) FTIR. To form the pellet, wood powders were collected by a single pass of sanding paper on the surface and mixed with KBr. Spectra were recorded between 400 and 4,000 cm−1. For selected samples, FTIR spectra were recorded by ATR FTIR. ATR–FTIR does not allow for near-surface averaging with regards to chemical composition, so in order to target plasma-elicited changes in surface lignin content (see below), the ATR probe was consistently placed in lignin-rich (late wood) area of the substrate. For all cases, background spectra were used to compensate for absorption by the ambient environment and spectra were adjusted to correct the baseline.
A VG ESCALAB mark II X-ray Photelectron Spectrometer (XPS) with a 206 W Mg X-ray source was used to determine the atomic composition of the near surface. The irradiated area was approximately 1 mm2. A general survey between 0 and 1,200 eV was performed to determine the C, O, and N atom compositions. High-resolution spectra of the C1s and O1s peaks were also collected. Each were de-convoluted into four peaks for C1s (C1–C4) and two peaks for O1s (O1 and O2), as it is usually done for cellulosic materials (Carlsson and Ström 1991; Gray 1978). When analyzing insulating samples by XPS, it is a common practice to shift the binding energy of the peak of interest as means of compensating for charge accumulation. In our case, the spectral convolution of the four C1s peaks complicated the selection of a reference peak. The effects of substrate charging were avoided by fixing the energy shift between each peak (C1–C4) when fitting the curve. Curve fitting to a Gaussian/Lorentzian function was performed manually with the CasaXPS software. For each peak, the full width at half maximum (2.0 eV) and the ratio between Gaussian and Lorentzian functions was the same. A general survey was performed both before and after the measurements to ensure the samples were not degraded by the X-ray photons.
Treatments of wood samples in other systems
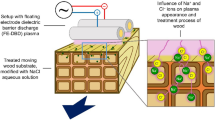
To more closely examine the role of UV irradiation in the wood modification dynamics, wood samples were exposed to the late afterglow of a N2–O2 plasma sustained by a propagating electromagnetic surface wave at 2.45 GHz. A schematic of the set-up illustrating the microwave plasma, early afterglow, and late afterglow regions is shown in Fig. 2. As described in Ref. Boudam et al. (2007), this set-up was optimized for UV emission from the NOγ (200–300 nm) and the NOβ (300–400 nm) systems for sterilization applications. In this work, the pressure was fixed to 2 Torr. The N2 and O2 mass flow rates were 1,000 and 11 SCCM (standard cubic centimeters per minute at STP), yielding an O2 relative concentration of 1 %. To isolate the effect of UV photons from other species generated in the microwave plasma, early afterglow, and late afterglow regions, selected wood substrates exposed to the late afterglow were enclosed in a UV-transparent envelope.
Schematic view of the low-pressure N2–O2 plasma source illustrating the main plasma region sustained by microwave electromagnetic field, the early afterglow (also called pink afterglow because the emission essentially results from the first negative system of N2 + in the 325–590 nm range), and the late afterglow (characterized by an orange–yellow color coming from the first positive system of N2 in the 0.5–1 μm range). Both afterglows are linked to the N2 vibration–vibration pumping mechanism occurring in the main plasma region and creating highly energetic vibrational states that are pushed downstream by the high gas flows. These energetic vibrational states can collide to form N2(A) and N2(a′) metastables and then ion–electron pairs by associative ionization reactions. Positive ion densities in the late afterglow of N2–O2 microwave plasmas are typically much below 107 cm−3 (Boudam et al. 2007; Ferreira et al. 2014), which ensures minimal ion-induced damage. (Color figure online)
As discussed below, in DBD systems open to ambient air, species generated in the nominally pure N2 discharge interact with surrounding O2 to form a number of species, including oxygen atoms and ozone. These species are also generated in the reduced-pressure N2–O2 plasma (Kutasi et al. 2008). To more clearly examine the role of ozone in the wood modification dynamics, treatments were also performed in an ozone generator, using an atmospheric pressure corona discharge supplied by a 4 L min−1 O2 flow rate. In the conditions investigated, the ozone generator provides an O3 concentration of approximately 4,000 ppm. Samples were exposed to ozone for durations of 5–15 min.
Experimental results and discussion
Dynamic water contact angle measurements
Dynamic water contact angle measurements from freshly-sanded sugar maple substrates exposed to the flowing afterglow of the N2 DBD open to ambient air are shown in Fig. 3. Water wetting properties after treatment exhibit a stark change from the interactions observed between water droplets and untreated sugar maple surfaces. Specifically, an accelerated rate is observed in the decrease of the dynamic contact angle. Considering that sugar maple is a porous and rough material, the dynamic contact angle can be measured while enough of the droplet remains above the surface. For this reason, the data displayed in Fig. 3 reflect only the portion of the data collected while the droplet angle exceeded the minimum threshold of the goniometer instrument.
Influence of the number of passes to the flowing afterglow of the N2 DBD open to ambient air on the wettability with water of sugar maple samples. The results are presented as a function of time after droplet application. For clarity, error bars (corresponding to one standard deviation of measurements taken over different areas of the wood sample) are only shown for untreated samples
The connection to the plasma treatment itself is made clear in Fig. 3, by observing the response of the surface to various doses of active plasma species. The term dose is used, reflecting the repeated temporary exposure of the wood sample to active species that are produced in the DBD. It is these active species that are understood to elicit the change in the surface. Considering the procedure of passing the sample beneath a flowing afterglow, it cannot be considered that the treatment was continuously applied for a total treatment time, given by the time per exposure multiplied by the number of passes. As shown in Fig. 3, there appears to be a “threshold” number of passes beyond which the treatment results in hydrophilization. This step change in the wettability of the wood surface, from properties similar to untreated surfaces to those of the hydrophilized surface, reveals important information about the DBD treatment process. A thermal effect cannot explain the change, since the surface temperature of the substrate could not increase with the number of passes. This is a result of the “long” time required by the plasma apparatus between passes. For this reason, the surface modification cannot be attributed to a thermally-activated volatilization of non-polar wood extractives, which under certain circumstances could have also produced a similar step change. Instead, it reinforces the idea that samples are treated dose-wise, since the surfaces were allowed to relax completely between passes.
For porous and rough substrates such as wood, hydrophilization comprises a combined effect of penetration and spreading of the deposited water droplet. Each type of surface treatment will produce a characteristic change in these two effects. The curves plotted in Fig. 4 illustrate the change in droplet spreading (baseline width) and droplet volume for as-prepared and plasma-treated sugar maple samples. These values were calculated from dynamic droplet images using the FTA200 software package. While the baseline width for untreated samples steadily increases with time after droplet application, measurements show an interesting difference resulting from plasma treatment. At first, the droplet spreads to a greater extent, which clearly indicates that the surface of the wood sample was hydrophically-activated. Slightly before the rate of penetration decreases (close to 0.5 s after droplet application), the baseline width begins to decrease, as a result of the droplet absorption. This is corroborated by the curves for droplet volume that show a dramatic change in the rate of penetration into the porous wood after plasma treatment. Such increase of the penetration rate clearly indicates either a hydrophilic activation of the internal wood surfaces or an increase in the free volume that would result from plasma etching of wood components (Jamali and Evans 2010). Although the type of volumetric effect (chemical or physical) cannot be concluded from this set of data, the presence of a volumetric modification implicates the role of long-lived species emanating from the plasma, capable of penetrating the porous matrix of the wood sample. For this reason, particular attention has been paid to stable and metastable atomic and molecular species when determining the likely causes of hydrophilization (see below).
Surface chemical analysis by FTIR and XPS
Figure 5 compares transmission FTIR spectra in the range between 2,500 and 4,000 cm−1 from as-prepared and plasma-treated sugar maple samples (10 passes). To emphasize the change in relative heights between the two prominent bands, the absorbance values were normalized relative to their respective values at 3,460 cm−1. In both samples, a large OH band is observed between 3,000 and 3,800 cm−1 and CHx bands (a convolution of CH, CH2, and CH3 bands) are found between 2,800 and 3,000 cm−1. A decrease of the OH/CHx area ratio (of ~30 %) can be observed after treatment, from 9.4 on untreated substrates to 6.6 for plasma-treated substrates (see Table 1). Furthermore, a pronounced sharpening of the two most prominent peaks of the CHx bands is observed at 2,850 and 2,915 cm−1, corresponding to CH2 stretching vibrations. When examining hardwood samples in transmission FTIR, these changes are consistent with increased lignin content (Faix 1991; Papp et al. 2005; Rodrigues et al. 1998). DBD treatment also increases the prominence of fringe structures between 3,800 and 3,900 cm−1 caused by adsorbed water (Al-Abadleh and Grassian 2003; Foster et al. 2002; Papp et al. 2004). These are direct indicators of surface hydrophilization. Environmental factors are not considered to be significant in affecting the signal, since the spectral measurements were controlled for environmental humidity and pellet preparation method.
FTIR transmission spectra (KBr pellet) for untreated and treated sugar maple. Peak sharpening in CH-stretching peaks and decreased relative height of OH-band is consistent with increased lignin content (Faix 1991; Papp et al. 2005; Rodrigues et al. 1998) and the formation of lignin precipitates (Rinne et al. 2005; Koljonen et al. 2004; Boeriu et al. 2004). Water vapor signal is characteristic of adsorbed water (Papp et al. 2005), and demonstrates hydrophilization of the wood surface
Another important segment of the transmission FTIR spectrum is presented in Fig. 6. The absorbance spectra are presented for the same samples as in Fig. 5 before and after treatment, in the range from 500 to 2,000 cm−1. To emphasize the relative change in peak heights for the numerous features of the complex absorbance pattern, the peaks are normalized at 1,047 cm−1, corresponding to the polysaccharide C–O stretch in cellulose (Rodrigues et al. 1998; Rinne et al. 2005; Zhbankov et al. 2002). An increase in absorbance intensity for peaks between 1,330 and 1,740 cm−1 is observed, indicating an increase in lignin composition (the specific lignin peak locations are listed in Table 2, with comparison to literature values and band assignments).
The increased lignin content after plasma treatment deduced from the two transmission FTIR spectra displayed in Figs. 5 and 6 could possibly be attributed to the formation of lignin precipitates (Rinne et al. 2005; Koljonen et al. 2004). This occurs by chemical modification of the lignin in wood, making well-ordered precipitates on exposed surfaces (Koljonen et al. 2004; Gilli et al. 2011). The peak sharpening in the unsaturated alkyl stretch seen in Fig. 5 could also indicate the formation of well-ordered lignin precipitates (Rinne et al. 2005; Pandey 1999). Measurements on pulp by atomic force microscopy have indicated that the dimensions of the precipitates are in the 10–100 nm range. The visco-elastic properties and unavoidable surface roughness of bulk wood samples makes AFM imaging, and thus visual confirmation of precipitates, a highly difficult task. Lignin precipitation is the first stage of the delignification process (Gilli et al. 2011; Maximova et al. 2004), which is typically executed by either chemical or ozone bleaching (Rinne et al. 2005; Pandey 1999; Mamleeva et al. 2013; Mamleeva et al. 2011; Mamleeva et al. 2012; Schorr et al. 2014; Sealey and Ragauskas 1998). In the case of ozone treatment, specifically, lignin precipitates in response to reactive scavenging of ether bonds that hold the lignin to the cellulosic wood structure (Gilli et al. 2011). The reaction produces an irreversible decrease in oxygen composition that can be measured by surface sensitive techniques, such as XPS (Haensel et al. 2009; Opila et al. 2009; Sinn et al. 2001). Although cellulose and hemicelluloses are also susceptible to oxidation under such conditions, reactive scavenging of ether bonds in lignin is energetically favored over the oxidation of OH groups in cellulose and hemicellulose (Gilli et al. 2011).
In addition to the OH/CHx ratios determined from transmission FTIR spectra, Table 1 also compares the chemical composition of the sugar maple surface measured by XPS before and after plasma treatment. The surface nitrogen concentration increases only marginally after treatment, making the grafting of N atoms on the surface during exposure to the N2 DBD flowing afterglow open to ambient air unlikely. A decrease of the surface oxygen concentration can be observed after the treatment, accompanied by an increase in the surface carbon concentration. The ratio of oxygen-to-carbon signal intensity (O/C) decreases from 0.47 to 0.39 (or approximately 17 %), which is again consistent with lignin precipitation.
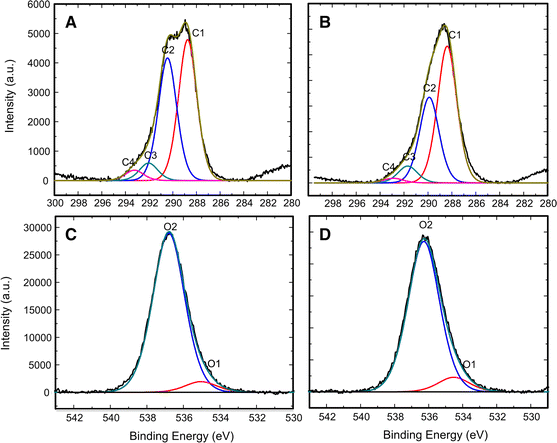
High-resolution XPS spectra were collected between 275 and 300 eV, corresponding to the C1s peak. The peak can be de-convoluted into four constituent peaks (C1–C4) representing different oxygen-containing chemical environments (Gray 1978). In the de-convolution presented in Fig. 7, C1 corresponds to carbon atoms bonded in C–C or C–H bonds, C2 is produced by carbon bound to a single oxygen, C3 by diether carbonyl bonds, and C4 results from carboxyl groups. Percent composition of each peak and the energy shift from the C1 sub-peak for untreated and DBD-treated sugar maple wood samples are shown in Table 3. A decrease of the C2 sub-peak after treatment is accompanied by an increase in C1 intensity. This indicates not only that fewer oxygenated C atoms remain on the surface after plasma treatment, but also that the change in surface oxygen content is focused on the reduction of single bonded oxygen-carbon groups. Table 3 further shows that the relative position between the C2 and C1 sub-peaks decreases from 1.7 to 1.5 eV. This is explained by a decrease of the ether bond function (C–O–C), which is observed at a higher binding energy than hydroxyl bonds (C–O–H) (López et al. 1991). Similar analysis of the O1s peak served to corroborate the changes seen in the C2 sub-peak. The O1s peak was de-convoluted by combining the O1 sub-peak, representing double-bonded oxygen (C=O), and the O2 sub-peak, representing single-bonded oxygen (C–O) (Sun et al. 2010). Table 3 show a measureable decrease of the O2 sub-peak after plasma treatment, corresponding to a reduced presence of single-bonded oxygen in treated samples. Both of these observations corroborate the information provided by FTIR, and confirm that the breaking of ether bonds is responsible for the increased signature of lignin in plasma-treated sugar maple wood samples.
Plasma characterization
The modification of sugar maple samples, as described above, can be induced by a number of species either generated directly in the DBD and pushed downstream by the high gas flows or produced following the interaction of the flowing afterglow of the DBD with ambient air. In this context, careful characterisation of the plasma by OES measurements was performed to determine the nature of plasma-generated species interacting with wood samples in the flowing afterglow. Figure 8 shows the OES spectrum from the open-air DBD operated in N2. Similarly to other spectra reported in literature for N2 DBDs [see, for example, Ref. Gherardi et al. (2000)], the most intense emission is due to the second positive system of N2 (C3Πu–B3Πg) between 300 and 500 nm (green and blue rovibrational bands in Fig. 8).
The emission from the second positive system of N2 was used to determine the temperature of neutral species interacting with the wood substrates. More precisely, the rotational temperature (assuming Boltzmann distribution of N2 rotational states) was calculated using the emission from the rovibrational band N2 (C3Πu, ν′ = 0–B3Πg, ν″ = 2) at 380 nm. This approach provides reasonable estimate of the neutral gas temperature provided the emitting N2(C) states are created by electron-impact excitation on ground state N2(X) such that the rovibrational structure of ground-state molecules is transferred “intact” into the excited state. In N2 DBDs, N2(C) states are, as assumed, mainly populated by electron-impact excitation on ground-state N2(X). High-resolution OES spectra in the 380 nm range were thus compared to the predictions of a model using the rotational temperature as the only adjustable parameter (Donnelly and Malyshev 2000). The results are shown in Fig. 9. The best fit was obtained for a rotational temperature of 322 K, which is comparable to the values reported in the literature under similar conditions (Poenariu et al. 2006). This neutral gas temperature being close to room temperature and much lower than the temperatures required to induce significant thermally-activated change in wood (Podgorski and Roux 1999), wood modification due to heat transfer during each single exposure to the flowing afterglow can reasonably be discarded.
The nature and number density of species generated in the DBD operated in nominally pure N2 were estimated using a collisional-radiative model coupled a Boltzmann code. This model was originally developed to describe atmospheric-pressure N2 plasmas sustained by microwave electromagnetic fields (Petrov et al. 2000). Steady-state particle balance equations are solved together for six excited states of N2,4 excited states of atomic nitrogen, and 4 ionized states of atomic and molecular nitrogen. The reactions considered in this model include elastic scattering, electron-impact excitation and de-excitation, stepwise excitation, radiative transitions, Penning ionization, three-body and dissociative recombination, along with the rotational and vibrational kinetics of N2. The rotational temperature and vibrational temperature (assuming Boltzmann distribution of rotational and vibrational states) of ground state N2(X) are used as input parameters to determine the number density of all species included in the model as well as the electron energy distribution function. While the rotational temperature was already determined from the high-resolution OES spectrum displayed in Fig. 9, the vibrational energy distribution function was analyzed from the Δν = −3 sequence (blue rovibrational bands in Fig. 8). As shown in Fig. 10, the distribution can be well-fitted by a straight line on a semi-log scale, confirming Boltzmann distribution of vibrational states characterized by a vibrational temperature of 1,920 K.
Vibrational energy distribution function of N2(C) states calculated for the Δν = −3 sequence (blue rovibrational bands in Fig. 8 and inset). Distribution follows a straight line on a semi-log scale, indicating Boltzmann distribution characterized by a vibrational temperature of 1,920 K
The predictions of the model for a nominally pure N2 plasma using the cycle-averaged values of rotational and vibrational temperatures determined above are presented in Table 4. For simplicity, only the non-radiative states are shown. It can be seen that the electron density is in the 108 cm−3 range with an electron temperature of about 1 eV, which is comparable to the values reported in literature for N2 DBDs (Poenariu et al. 2006). Significant concentrations of N atoms (1016 cm−3), positive ions (dominant positive ion is N4 + with a population of 9 × 107 cm−3), N2(A) metastables (1014 cm−3), and N2(a′) metastables (108 cm−3) are also predicted by the model.
The plasma properties presented in Table 4 corresponds to those inside the DBD source; those close to the substrate surface obviously differ greatly due to the ambient air configuration. Since a detailed collisional-radiative model accounting for the interaction of a N2 DBD with ambient air is not yet available, simple calculations were performed to estimate the nature of the species produced in the N2 DBD and capable of reaching the wood substrate placed in the flowing afterglow. In this context, the effective lifetime of each species was compared to the time required to reach the wood surface under a gas flow rate of 50 L min−1 (average speed at the DBD outlet of about 300 cm s−1). For a DBD-to-wood substrate distance of 0.3 cm, the downstream time is estimated to 10−3 s. To calculate the effective lifetime, τ eff , of a given species in the flowing afterglow, the dominant loss pathway must be determined. For N atoms, assuming three-body gas phase recombination (on N2) as the major loss mechanism (Kossyi et al. 1992), τ eff = 3×10−4 s. This value being lower than the downstream time, most of the N atoms created in the DBD region are lost before interacting with the wood sample, which supports the very low N uptakes recorded by XPS (see Table 1). For the metastable states [N2(A) and N2(a′)], assuming that losses are dominated by quenching on N2 (Kossyi et al. 1992) yields τ eff = 1.4 × 10−2 s for N2(A) and τ eff = 2×10−7 s for N2(a′). Over the range of conditions investigated, N2(A) states are thus expected to play a very important role either directly following their de-excitation by collisions with the wood substrate (transfer of internal energy ~6.2 eV) or indirectly through the production of other active species in the flowing afterglow. In particular, N2(A) states in DBDs operated in O2-containing gases are known to produce large quantities of atomic oxygen through the reaction
and thus ozone by
where M is a third body for associative reactions (gas phase and substrate atoms and molecules) (Šimek et al. 2012). Finally, for N4 + ions, assuming dissociative recombination as the dominant loss term (Kossyi et al. 1992), τ eff = 3×10−2 s. Even if this value is comparable to the downstream time, the number density of positive ions is much lower than the one of N2(A) such that the latter species is expected to play a more prominent role.
In the DBD system investigated in this study, strong emission from the NOγ system (A2Σ+–X2Πg) between 200 and 300 nm (red rovibrational bands in Fig. 8) as well as emission from O(1s)N2 at 558 nm (orange rovibrational bands in Fig. 8) were also observed in the OES spectrum. Such emission bands in N2 DBDs indicate the presence of oxygen; a feature obviously ascribed to the open-air configuration of our system. Emission from NO in the 200–300 nm range corresponds to photon energies in the 4.1–6.2 eV range, which are sufficient to cause chemical changes in wood surfaces (Papp et al. 2005). Therefore, UV irradiation could play a very important role in the wood modification dynamics. In combination with N2(A) metastables, these UV photons with energy hc/λ (where h is the Planck constant, c is the speed of light, and λ is the wavelength) could also be enrolled in the production of oxygen atoms and ozone close to the wood substrate due to the well-known photo-dissociation reaction O 2 + hc/λ → 2O.
Role of plasma-generated UV irradiation
UV photons are known to alter the chemistry of macromolecular surfaces and to be strongly implicated in wood ageing (Papp et al. 2005). For this reason, UV was examined as a potential cause of the surface activation evidenced in Figs. 3 and 4. In this context, wood samples were exposed to the late afterglow of a low-pressure N2–O2 plasma (see Fig. 2). This is a means of exposing wood samples to a source of characteristic short-wave UV emission from NO, which was also observed in the N2 DBD open to ambient air (see Fig. 8). Figure 11 shows the dynamic water contact angle of freshly-sanded sugar maple samples after treatment for a period of 5 min. The initial contact angle decreases to 20° and the total absorption time is approximately 0.25 s. The isolated effect of UV-treatment on wettability with respect to other species present in the late afterglow was analyzed by placing wood samples in a UV-transparent enclosure within the plasma chamber. As shown in Fig. 11, UV irradiation alone did not result in any measureable deviation in wetting behaviour from as-prepared sugar maple surfaces. This indicates that UV photons are not directly involved in the plasma-induced hydrophilization. In the open-air configuration of the N2 DBD, these energetic photons rather participate [along with N2(A) metastables] in the formation of other active species, in particular atomic oxygen and ozone (see below).
Role of plasma-generated oxygen atoms and ozone
As mentioned above, the combined presence of long-lived N2(A) metastable states and UV photons is responsible for the creation of a number of species, including atomic oxygen and ozone in the flowing afterglow of N2 DBDs open to ambient air. Although both species are potent oxidizers, O atoms are more aggressive than molecular ozone (Mamleeva et al. 2012). The exposure to each in isolation is necessary to confirm that both species can induce wood modification, as well as to assess the relative activity of each individual species.
In the late afterglow of the low-pressure N2–O2 plasma, the number density of O atoms is expected to be significant [~1014 cm−3 (Boudam et al. 2007)]. However, because of the reduced-pressure conditions, three body gas-phase collisions leading to the production of ozone by reaction (2) are not significant. In this system, O atoms therefore drive the hydrophilization of sugar maple samples (total absorption time is 0.25 s, see Fig. 11). Isolated effect of ozone exposure was realized by treatment in an ozone generator providing O3 number density of ~1017 cm−3 (4,000 ppm at atmospheric pressure). Figure 12 shows the effect of ozone treatment on dynamic water contact angle on sugar maple samples after treatments of 5 and 15 min. Sugar maple samples were hydrophilized for both treatments, with the effect increasing progressively with treatment duration.
FTIR–ATR measurements of samples exposed to ozone and oxygen atoms (late afterglow of the low-pressure N2–O2 plasma) are shown in Fig. 13. For both samples, the results are similar to what was seen in Fig. 6, with lignin peaks between 900 and 1,800 cm−1 absorbing to a greater extent after treatment. This is again consistent with increased lignin content and lignin precipitation.
Although both ozone and oxygen atoms hydrophilize wood surfaces and yield to such precipitation, the effect of ozone treatment is much less severe, despite the much higher O3 number density in the ozone generator with respect to the O number density in the late afterglow of the low-pressure N2–O2 plasma. This confirms that atomic oxygen is more aggressive, particularly for reactive etching of the ligno-cellulosic linkage. This higher activity would also impact the increase of the free volume that would result from plasma etching of wood components (Jamali and Evans 2010). Although the number densities of both species cannot be precisely determined in the flowing afterglow of the N2 DBD open to ambient air, nonetheless both ozone and oxygen atoms are expected to play an important role in the plasma-wood modification. However, while the effect of ozone is clearly significant, the short exposure times used during DBD treatments and the dose-wise exposure method confers greater importance to the more aggressive species present, most likely O atoms.
Conclusions
The combined findings of surface characterization by spectroscopic techniques as well as dynamic water contact angle measurements have clearly identified a strong hydrophilization of sugar maple wood surfaces after exposure to the flowing afterglow of N2 DBDs open to ambient air. The activation process is determined to be a multi-step modification of the topmost surface and the near-surface region by plasma-generated species. N2(A) metastable species and UV photons from the NOγ system, both produced in significant quantities in the DBD, interact with oxygen due to ambient air configuration to produce ozone and atomic oxygen. These species yield to an enhanced lignin content, the possible development of lignin precipitates, and could also contribute to surface etching of cellulose and hemicelluloses.
Further research should be performed for practical applications to be developed. For example, a study of the adhesion improvement with one or several coatings after the DBD treatment could be performed. Questions it should answer are (1) can the strong hydrophilization lead to an improvement of the adhesion and (2) can the observed lignin degradation improve chemical bonding between the surface and the coating, by means of cross-linking or any other mechanism.
References
Acda MN, Devera EE, Cabangon RJ, Ramos HJ (2011) Effects of plasma modification on adhesion properties of wood. Int J Adhes Adhes 32(2012):70–75
Akishev Y, Grushin M, Dyatko N, Kochetov I, Napartovich A, Trushkin N, Minh Duc T, Descours S (2008) Studies on cold plasma–polymer surface interaction by example of PP- and PET-films. J Phys D Appl Phys 41(23):235203
Al-Abadleh HA, Grassian VH (2003) FT-IR study of water adsorption on aluminum oxide surfaces. Langmuir 19:341–347
Asandulesa M, Topala I, Dumitrascu N (2010) Effect of helium DBD plasma treatment on the surface of wood samples. Holzforschung 64(2):223–227
Avramidis G, Hauswald E, Lyapin A, Militz H, Viöl W, Wolkenhauer A (2009) Plasma treatment of wood and wood-based materials to generate hydrophilic or hydrophobic surface characteristics. Wood Mat Sci Eng 4(1–2):52–60
Aydin İ (2004) Activation of wood surfaces for glue bonds by mechanical pre-treatment and its effects on some properties of veneer surfaces and plywood panels. Appl Surf Sci 233(1–4):268–274
Bekhta P, Niemz P (2003) Effect of high temperature on the change in color, dimensional stability and mechanical properties of spruce wood. Holzforschung 57:539–546
Boeriu CG, Bravo D, Gosselink RJA, van Dam JEG (2004) Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crops Prod 20:205–218
Boudam MK, Saoudi B, Moisan M, Ricard A (2007a) Characterization of the flowing afterglows of an N2–O2 reduced-pressure discharge: setting the operating conditions to achieve a dominant late afterglow and correlating the NOβ UV intensity variation with the N and O atom densities. J Phys D Appl Phys 40(6):1694–1711
Boudam M, Saoudi B, Moisan M, Ricard A (2007b) Characterization of the flowing afterglows of an N2–O2 reduced-pressure discharge: setting the operating conditions to achieve a dominant late afterglow and correlating the NOβ UV intensity variation with the N and O atom densities. J Phys D Appl Phys 40(6):1694
Busnel F, Blanchard V, Prégent J, Stafford L, Riedl B, Blanchet P, Sarkissian A (2010) Modification of sugar maple (Acer saccharum) and black spruce (Picea mariana) wood surfaces in a dielectric barrier discharge (DBD) at atmospheric pressure. J Adhes Sci Technol 24(8–10):1401–1413
Carlsson CMG, Ström G (1991) Reduction and oxidation of cellulose surfaces by means of cold plasma. Langmuir 7:2492–2497
Cotrim AR, Ferraz A, Gonçalves AR, Silva FT, Bruns RE (1999) Identifying the origin of lignins and monitoring their structural changes by means of FTIR-PCA and -SIMCA. Bioresour Technol 68:29–34
Custódio J, Broughton J, Cruz H, Winfield P (2009) Activation of timber surfaces by flame and corona treatments to improve adhesion. Int J Adhes Adhes 29(2):167–172
Denes AR, Mandla A, Tshabalala RR, Denes F, Young RA (1999) Hexamethyldisiloxane-plasma coating of wood surfaces for creating water repellent characteristics. Holzforschung 53:318–326
Donnelly VM, Malyshev MV (2000) Diagnostics of inductively coupled chlorine plasmas: measurements of the neutral gas temperature. Appl Phys Lett 77(16):2467
Egitto FD, Matienzo LJ (1994) Plasma modification of polymer surfaces for adhesion improvement. IBM J Res Dev 38(4):423–439
Faix O (1991) Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung: Int J Biol Chem Phys Technol Wood 45:21–28
Ferreira JA, Stafford L, Leonelli R, Ricard A (2014) Electrical characterization of the flowing afterglow of N2 and N2/O2 microwave plasmas at reduced pressure. J Appl Phys 115(16):163303
Foster M, Furse M, Passno D (2002) An FTIR study of water thin films on magnesium oxide. Surf Sci 502–503:102–108
Gherardi N, Gouda G, Gat E, Ricard A, Massines F (2000) Transition from glow silent discharge to micro-discharges in nitrogen gases. Plasma Sour Sci Technol 9:340–346
Gilli E, Schmied F, Diebald S, Horvath AT, Teichert C, Schennach R (2011) Analysis of lignin precipitates on ozone treated kraft pulp by FTIR and AFM. Cellulose 19:249–256
Gray D (1978) The surface analysis of paper and wood fibres by ESCA (I). Application to cellulose and lignin. Cell Chem Technol 12:9–23
Haensel T, Comouth A, Lorenz P, Ahmed SI-U, Krischok S, Zydziak N, Kauffmann A, Schaefer JA (2009) Pyrolysis of cellulose and lignin. Appl Surf Sci 255:8183–8189
Hegemann D, Brunner H, Oehr C (2003) Plasma treatment of polymers for surface and adhesion improvement. Nucl Instrum Methods Phys Res Sect B 208:281–286
Jamali A, Evans PD (2010) Etching of wood surfaces by glow discharge plasma. Wood Sci Technol 45(1):169–182
Klarhöfer L, Viöl W, Maus-Friedrichs W (2010) Electron spectroscopy on plasma treated lignin and cellulose. Holzforschung 64(3):331–336
Koljonen K, Österberg M, Kleen M, Fuhrmann A, Stenius P (2004) Precipitation of lignin and extractives on kraft pulp: effect on surface chemistry, surface morphology and paper strength. Cellulose 11:209–224
Kossyi I, Kostinsky AY, Matveyev A, Silakov V (1992) Kinetic scheme of the non-equilibrium discharge in nitrogen–oxygen mixtures. Plasma Sour Sci Technol 1(3):207
Kutasi K, Saoudi B, Pintassilgo CD, Loureiro J, Moisan M (2008) Modelling the low-pressure N2–O2 plasma afterglow to determine the kinetic mechanisms controlling the UV emission intensity and its spatial distribution for achieving an efficient sterilization process. Plasma Process Polym 5(9):840–852
Levasseur O, Stafford L, Gherardi N, Naudé N, Blanchard V, Blanchet P, Riedl B, Sarkissian A (2012) Deposition of hydrophobic functional groups on wood surfaces using atmospheric-pressure dielectric barrier discharge in helium-hexamethyldisiloxane gas mixtures. Plasma Process Polym 9(11–12):1168–1175
Liu Q, Wang S, Zheng Y, Luo Z, Cen K (2008) Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J Anal Appl Pyrol 82:170–177
López GP, Castner DG, Ratner BD (1991) XPS O 1 s binding energies for polymers containing hydroxyl, ether, ketone and ester groups. Surf Interface Anal 17:267–272
Mahlberg R, Niemi HE-M, Denes F, Rowell RM (1998) Effect of oxygen and hexamethyldisiloxane plasma on morphology, wettability and adhesion properties of polypropylene and lignocellulosics. Int J Adhes Adhes 18:283–297
Mamleeva NA, Kharlanov AN, Fionov AV, Lunin VV (2011) Ozonation of deciduous wood in the presence of hydrogen peroxide. Russ J Phys Chem A 85:1729–1736
Mamleeva NA, Kharlanov AN, Lunin VV (2012) Delignification of deciduous wood under the action of hydrogen peroxide and ozone. Russ J Phys Chem A 87:28–34
Mamleeva NA, Abrosimova GE, Kharlanov AN, Lunin VV (2013) Transformation of wood during ozonization in the presence of hydrogen peroxide. Russ J Phys Chem A 87:1102–1107
Maximova N, Österberg M, Laine J, Stenius P (2004) The wetting properties and morphology of lignin adsorbed on cellulose fibres and mica. Colloids Surf A 239:65–75
Naudé N, Cambronne JP, Gherardi N, Massines F (2005) Electrical model and analysis of the transition from an atmospheric pressure Townsend discharge to a filamentary discharge. J Phys D Appl Phys 38(4):530–538
Opila R, Ertas G, Popescu C-M, Tibirna C-M, Vasile C (2009) XPS characterization of naturally aged wood. Appl Surf Sci 256:1355–1360
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975
Papp G, Preklet E, Košıková B, Barta E, Tolvaj L, Bohus J, Szatmári S, Berkesi O (2004) Effect of UV laser radiation with different wavelengths on the spectrum of lignin extracted from hard wood materials. J Photochem Photobiol, A 163:187–192
Papp G, Barta E, Preklet E, Tolvaj L, Berkesi O, Nagy T, Szatmári S (2005) Changes in DRIFT spectra of wood irradiated by UV laser as a function of energy. J Photochem Photobiol A 173:137–142
Petrov GM, Matte JP, Peres I, Margot J (2000) Numerical modeling of a He–N2 capillary surface wave discharge at atmospheric pressure. Plasma Chem Plasma Process 20(2):183–208
Podgorski L, Roux M (1999) Wood modification to improve the durability of coatings. Surf Coat Int 12:590–596
Podgorski L, Chevet B, Onic L, Merlin A (2000) Modification of wood wettability by plasma and corona treatments. Int J Adhes Adhes 20:103–111
Poenariu V, Wertheimer MR, Bartnikas R (2006) Spectroscopic diagnostics of atmospheric pressure helium dielectric barrier discharges in divergent fields. Plasma Process Polym 3(1):17–29
Rehn P, Viöl W (2003) Dielectric barrier discharge treatments at atmospheric pressure for wood surface modification. Holz als Roh- und Werkstoff 61:145–150
Rinne KT, Boettger T, Loader NJ, Robertson I, Switsur VR, Waterhouse JS (2005) On the purification of α-cellulose from resinous wood for stable isotope (H, C and O) analysis. Chem Geol 222:75–82
Rodrigues J, Faix O, Pereira H (1998) Determination of lignin content of Eucalyptus globulus wood using FTIR spectroscopy. Holzforschung: Int J Biol Chem Phys Technol Wood 52:46–50
Scholze B, Meier D (2001) Characterization of the water-insoluble fraction from pyrolysis oil (pyrolytic lignin). Part I. PY–GC/MS, FTIR, and functional groups. J Anal Appl Pyrol 60:41–54
Schorr D, Diouf PN, Stevanovic T (2014) Evaluation of industrial lignins for biocomposites production. Ind Crops Prod 52:65–73
Sealey J, Ragauskas AJ (1998) Residual lignin studies of laccase-delignified kraft pulps. Enzyme Microbial Technol 23:422–426
Šimek M, Pekárek S, Prukner V (2012) Ozone production using a power modulated surface dielectric barrier discharge in dry synthetic air. Plasma Chem Plasma Process 32(4):743–754
Sinn G, Reiterer A, Stanzl-Tschegg SE (2001) Surface analysis of different wood species using X-ray photoelectron spectroscopy (XPS). J Mater Sci 36:4673–4680
Sun Y, Royer M, Diouf PN, Stevanovic T (2010) Chemical changes induced by high-speed rotation welding of wood—application to two Canadian hardwood species. J Adhes Sci Technol 24(8–10):1383–1400
Toriz G, Gutiérrez MG, González-Alvarez V, Wendel A, Gatenholm P, Martínez-Gómez AD (2008) J Highly hydrophobic wood surfaces prepared by treatment with atmospheric pressure dielectric barrier discharges. J Adhes Sci Technol 22(16):2059–2078
Wolkenhauer A, Avramidis G, Hauswald E, Militz H, Viöl W (2008) Plasma treatment of wood–plastic composites to enhance their adhesion properties. J Adhes Sci Technol 22(16):2025–2037
Wolkenhauer A, Avramidis G, Hauswald E, Militz H, Viöl W (2009) Sanding vs. plasma treatment of aged wood: a comparison with respect to surface energy. Int J Adhes Adhes 29(1):18–22
Zhbankov RG, Firsov SP, Buslov DK, Nikonenko NA, Marchewka MK, Ratajczak H (2002) Structural physico-chemistry of cellulose macromolecules. Vibrational spectra and structure of cellulose. J Mol Struct 614:117–125
Acknowledgments
This work was supported by the National Science and Engineering Research Council (NSERC) of Canada through the strategic Project Grant and the discovery Grant Programs. The authors would also like to acknowledge Prof. Joëlle Margot for providing the collisional-radiative model for N2 plasmas at atmospheric pressure that was used in this work, Dr. Fabienne Poncin-Épaillard for the first set of FTIR measurements of plasma-treated wood samples, and Dr. Frédéric Busnel for experimental groundwork and preliminary measurements on wettability.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prégent, J., Vandsburger, L., Blanchard, V. et al. Determination of active species in the modification of hardwood samples in the flowing afterglow of N2 dielectric barrier discharges open to ambient air. Cellulose 22, 811–827 (2015). https://doi.org/10.1007/s10570-014-0496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0496-8