Abstract
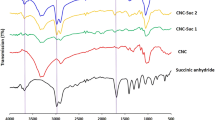
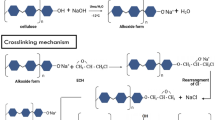
Cellulose nanocrystals (CNCs) prepared from cellulose fibre via sulfuric acid hydrolysis was used as an adsorbent for the removal of methylene blue (MB) from aqueous solution. The effects of pH, adsorbent dosage, temperature, ionic strength, initial dye concentration were studied to optimize the conditions for the maximum adsorption of dye. Adsorption equilibrium data was fitted to both Langmuir and Freundlich isotherm models, where the Langmuir model better described the adsorption process. The maximum adsorption capacity was 118 mg dye/g CNC at 25 °C and pH 9. Calculated thermodynamic parameters, such as free energy change (ΔG = −20.8 kJ/mol), enthalpy change (ΔH = −3.45 kJ/mol), and entropy change (ΔS = 0.58 kJ/mol K) indicates that MB adsorption on CNCs is a spontaneous exothermic process. Tunability of the adsorption capacity by surface modification of CNCs was shown by oxidizing the primary hydroxyl groups on the CNC surface with TEMPO reagent and the adsorption capacity was increased from 118 to 769 mg dye/g CNC.




Similar content being viewed by others
References
Acemioglu B (2004) Adsorption of congo red from aqueous solution onto calcium-rich fly ash. J Colloid Interface Sci 274:371–379
Akhlaghi SP, Berry RC, Tam KC (2013) Surface modification of cellulose nanocrystal with chitosan oligosaccharide for drug delivery applications. Cellulose 20:1747–1764
Azzam F, Heux L, Putaux J, Jean B (2010) Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 11:3652–3659
Beck S, Bouchard J, Berry R (2012) Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules 13:1486–1494
Bhatnagar A, Jain AK (2005) A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci 281:49–55
Cai Z, Kim J (2008) Characterization and electromechanical performance of cellulose–chitosan blend electro-active paper. Smart Mater Struct 17:1–8
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97
de Mesquita JP, Donnici CL, Pereira FV (2010) Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules 11:473–480
Deng H, Yang L, Tao G, Dai J (2009) Preparation and characterization of activated carbon from cotton stalk by microwave assisted chemical activation—application in methylene blue adsorption from aqueous solution. J Hazard Mater 166:1514–1521
Dogan M, Abak H, Alkan M (2008) Biosorption of methylene blue from aqueous solutions by hazelnut shells: equilibrium, parameters and isotherms. Water Air Soil Pollut 192:141–153
Doğan M, Alkan M, Türkyilmaz A, Özdemir Y (2004) Kinetics and mechanism of removal of methylene blue by adsorption onto perlite. J Hazard Mater 109:141–148
Dong XM, Kimura T, Revol JF, Gray DG (1996) Effects of Ionic strength on the phase separation of suspensions of cellulose crystallites. Langmuir 12:2076–2082
Gu J, Catchmark JM, Kaiser EQ, Archibald DD (2013) Quantification of cellulose nanowhiskers sulfate esterification levels. Carbohydr Polym 92:1809–1816
Gulnaz O, Kaya A, Matyar F, Arikan B (2004) Sorption of basic dyes from aqueous solution by activated sludge. J Hazard Mater 108:183–188
Gupta VK, Srivastava SK, Tyagi R (2000) Design parameters for the treatment of phenolic wastes by carbon columns (obtained from fertilizer waste material). Water Res 34:1543–1550
Gupta VK, Ali I, Saini VK, Gerven TV, Bruggen BVd, Vecasteele C (2005) Removal of dyes from wastewater using bottom ash. Ind Eng Chem Res 44:3655–3664
Gupta VK, Jain R, Mittal A, Mathur M, Sikarwar S (2007) Photochemical degradation of the hazardous dye safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Mittal A, Malviya A, Mittal J (2009) Adsorption of carmoisine a from wastewater using waste materials-bottom ash and deoiled soya. J Colloid Interface Sci 335:24–33
Gupta VK, Rastogi A, Nayak A (2010) Biosorption of nickel onto treated alga (Oedogonium hatei): application of isotherm and kinetic models. J Colloid Interface Sci 342:533–539
Gupta VK, Agarwal S, Singh P, Pathania D (2013a) Acrylic acid grafted cellulosic luffa cylindrical fiber for the removal of dye and metal ions. Carbohydr Polym 98:1214–1221
Gupta VK, Pathania D, Singh P, Rathore BS, Chauhan P (2013b) Cellulose acetate–zirconium (IV) phosphate nano-composite with enhanced photo-catalytic activity. Carbohydr Polym 95:434–440
Gupta VK, Pathania D, Singh P, Kumar A, Rathore BS (2014) Adsorptional removal of methylene blue by guar gum-cerium (IV) tungstate hybrid cationic exchanger. Carbohydr Polym 101:684–691
Gürses A, Karaca S, Doğar Ç, Bayrak R, Açıkyıldız M, Yalçın M (2004) Determination of adsorptive properties of clay/water system: methylene blue sorption. J Colloid Interface Sci 269:310–314
Habibi Y, Chanzy H, Vignon MR (2006) TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 13:679–687
Hai FI, Yamamoto K, Fukushi K (2007) Hybrid treatment systems for dye wastewater. Crit Rev Environ Sci Technol 37:315–377
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135:264–273
He X, Male KB, Nesterenko PN, Brabazon D, Paull B, Luong JHT (2013) Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl Mater Interfaces 5:8796–8804
Ho YS, McKay G (2003) Sorption of dyes and copper ions onto biosorbents. Process Biochem 38:1047–1061
Hunger K (2003) Industrial dyes: chemistry, properties, applications. Wiley, Weinheim
Husain Q (2006) Potential applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: a review. Crit Rev Biotechnol 26:201–221
Jain AK, Gupta VK, Bhatnagar A, Suhas (2003) Utilization of industrial waste products as adsorbents for the removal of dyes. J Hazard Mater 101:31–42
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466
Korzoun VI, Sokolov AA, Budyko MI, Voskresensky KP, Kalinin GP (1978) World water balance and water resources of the earth. UNESCO Press, Paris
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Adsorptive removal of malachite green dye from aqueous solution by bagasse fly ash and activated carbon-kinetic study and equilibrium isotherm analyses. Colloids and surfaces. A Physiochem Eng Asp 264:17–28
Marungrueng K, Pavasant P (2007) High performance biosorbent (Caulerpa lentillifera) for basic dye removal. Bioresour Technol 98:1567–1572
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresour Technol 97:512–521
Métivier-Pignon H, Faur-Brasquet C, Le Cloirec P (2003) Adsorption of dyes onto activated carbon cloths: approach of adsorption mechanisms and coupling of ACC with ultrafiltration to treat coloured wastewaters. Sep Purif Technol 31:3–11
Mittal A, Gupta VK, Malviya A, Mittal J (2008) Process development for the batch and bulk removal and recovery of a hazardous, water-soluble azo dye (metanil yellow) by adsorption over waste materials (bottom ash and de-oiled soya). J Hazard Mater 151:821–832
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343:463–473
Ozacar M, Sengil IA (2005) Adsorption of metal complex dyes from aqueous solutions by pine sawdust. Bioresour Technol 96:791–795
Peng BL, Dhar N, Liu HL, Tam KC (2011) Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective. Can J Chem Eng 89:1191–1206
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177:70–80
Rai HS, Bhattacharyya MS, Singh J, Bansal TK, Vats P, Banerjee UC (2005) Removal of dyes from the effluent of textile and dyestuff manufacturing industry: a review of emerging techniques with reference to biological treatment. Crit Rev Environ Sci Technol 35:219–238
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Saha P, Chowdhury S, Gupta S, Kumar I, Kumar R (2010) Assessment on the removal of malachite green using tamarind fruit shell as biosorbent. Clean-Soil Air Water 38:437–445
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Sharma P, Kaur H, Sharma M, Sahore V (2011) A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste. Environ Monit Assess 183:151–195
Široký J, Blackburn RS, Bechtold T, Taylor J, White P (2011) Alkali treatment of cellulose II fibres and effect on dye sorption. Carbohydr Polym 84:299–307
Sivasamy A, Sundarabal N (2011) Biosorption of an azo dye by Aspergillus niger and Trichoderma sp. Fungal Biomasses. Curr Microbiol 62:351–357
Unuabonah EI, Adebowale KO, Dawodu FA (2008) Equilibrium, kinetic and sorber design studies on the adsorption of aniline blue dye by sodium tetraborate-modified kaolinite clay adsorbent. J Hazard Mater 157:397–409
Wang S, Boyjoo Y, Choueib A, Zhu ZH (2005) Removal of dyes from aqueous solution using fly ash and red mud. Water Res 39:129–138
Wang S, Li H, Xu L (2006) Application of zeolite MCM-22 for basic dye removal from wastewater. J Colloid Interface Sci 295:71–78
Wong YC, Szeto YS, Cheung WH, McKay G (2003) Equilibrium studies for acid dye adsorption onto chitosan. Langmuir 19:7888–7894
Wong Y, Szeto Y, Cheung W, McKay G (2004) Adsorption of acid dyes on chitosan—equilibrium isotherm analyses. Process Biochem 39:695–704
Acknowledgments
Rasim Batmaz (RB) thanks the Ministry of National Education of the Republic of Turkey for supporting his research at University of Waterloo, Canada. RB wishes to acknowledge Parinaz Akhlaghi for several helpful discussion on this subject. The authors would also like to acknowledge FP Innovations and CelluForce for the supply of CNC for this research. This work is also supported by NSERC, CFI Canada.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Batmaz, R., Mohammed, N., Zaman, M. et al. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 21, 1655–1665 (2014). https://doi.org/10.1007/s10570-014-0168-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-014-0168-8