Abstract
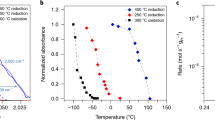
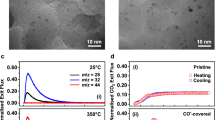
Quantitative measurement of the number of active surface sites on two-dimensional (2D) catalysts is one of the most crucial points in heterogeneous catalysis because it is used to determine the turnover frequency (TOF), which refers to the catalytic activity of model catalysts. However, because of the difficulty in identifying the effective active surface area on 2D heterogeneous catalysts, there is still the assumption that each metal atom is an active site. To shed light on these issues and to bridge the activity gaps between 2D and three-dimensional (3D) heterogeneous catalysts, we present an experimental approach that uses Pt nanoparticle (NP) arrays on a thin silicon wafer probed with CO pulse chemisorption, a widely used surface-sensitive technique, to determine the number of active sites and the area of the effective active surface. A Pt thin film and Pt NP arrays with two different NP sizes (i.e., 2.1 and 4.5 nm) were prepared as model systems for 2D catalysts. The effective active metal surface area determined using CO pulse chemisorption for these 2D catalysts is 53–79% of the apparent metal surface area that was obtained by measuring the surface area based on scanning electron microscopy images. This discrepancy between the active and apparent surface area is attributed to the presence of hydrocarbon contamination and organic capping layers on the catalysts. The results indicate that estimating the active sites of 2D catalysts by apparent surface area is reasonably in agreement with the number measured by chemisorption that is used to characterize 3D nanocatalysts. This experimental technique on 2D catalysts can be expected to provide information for extracting the true TOF of product molecules on 2D catalysts in gas-phase catalytic reactions.
Graphical Abstract





Similar content being viewed by others
References
Nørskov JK, Bligaard T, Hvolbaek B, Abild-Pedersen F, Chorkendorff I, Christensen CH (2008) Chem Soc Rev 37:2163–2171
Thomas JM, Thomas WJ (1997) Principles and practice of heterogeneous catalysis. VCH, New York
Tatibouët JM (1997) Appl Catal A Gen 148:213–252
Taylor HS (1925) Proc R Soc Lond Ser A 108:105–111
Kim SM, Qadir K, Seo B, Jeong HY, Joo SH, Terasaki O, Park JY, Park Y (2013) Catal Lett 143:1153–1161
Somorjai GA, McCrea KR, Zhu J (2002) Top Catal 18:3–4
Somorjai GA, Park JY (2008) Angew Chem Int Ed 47:9212–9228
Grunes J, Zhu J, Yang M, Somorjai GA (2003) Catal Lett 86:157–161
Park JY, Zhang Y, Joo SH, Jung Y, Somorjai GA (2012) Catal Today 181:133–137
Park JY, Zhang Y, Grass M, Zhang T, Somorjai GA (2008) Nano Lett 8:673–677
Park JY, Somorjai GA (2006) ChemPhysChem 7:1409–1413
Contreras AM, Grunes J, Yan X-M, Liddle A, Somorjai GA (2006) Top Catal 39:123–129
Somorjai GA, Park JY (2008) Top Catal 49:126–135
Burcham LJ, Briand LE, Wachs IE (2001) Langmuir 17:6164–6174
Jung C-H, Yun J, Qadir K, Naik B, Yun J-Y, Park JY (2014) Appl Catal B Environ 154–155:171–176
Ertl G (1991) Science 254:1750–1755
Falsig H, Hvolbæk B, Kristensen IS, Jiang T, Bligaard T, Christensen CH, Nørskov JK (2008) Angew Chem Int Ed 120:4913–4917
Chen MS, Cai Y, Yan Z, Gath KK, Axnanda S, Goodman DW (2007) Surf Sci 601:5326–5331
Song H, Rioux RM, Hoefelmeyer JD, Komor R, Niesz K, Grass M, Yang P, Somorjai GA (2006) J Am Chem Soc 128:3027–3037
Naik B, Moon SY, Oh S, Jung C-H, Park JY (2015) Catal Lett 145:930–938
Park JY (2011) Langmuir 27:2509–2513
Aliaga C, Park JY, Yamada Y, Lee HS, Tsung CK, Yang P, Somorjai GA (2009) J Phys Chem C 113:6150–6155
Schwab GM, Koller K (1968) J Am Chem Soc 90:3078–3080
Solymosi F. (1968) Catal Rev Sci Eng 1:233–255
Kim SM, Lee SJ, Kim SH, Kwon S, Yee KJ, Song H, Somorjai GA, Park JY (2013) Nano Lett 13:1352–1358
Broussard ME, Juma B, Train SG, Peng W-J, Laneman SA, Stanley GG (1993) Science 260:1784–1788
Mondal A, Jana NR (2015) RSC Adv 5:85196–85201
Kim SM, Park D, Yuk Y, Kim SH, Park JY (2013) Faraday Discuss 162:355–364
Acknowledgements
This work was supported by IBS-R004-G4.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10562_2016_1909_MOESM1_ESM.pdf
Supplementary material 1. Further details on the experimental section and additional characterization. TEM images and the size distribution of the 2.1 and 4.5 nm Pt NPs (Figure S1); XPS spectra of the Pt thin film and the Pt NP arrays supported on silicon before CO chemisorption (Figure S2); Pt oxidation states for three different Pt catalysts from XPS plots (Table S1). (PDF 307 KB)
Rights and permissions
About this article
Cite this article
Oh, S., Qadir, K. & Park, J.Y. Nature of Active Sites and Their Quantitative Measurement in Two-Dimensional Pt Metal Catalysts. Catal Lett 147, 39–45 (2017). https://doi.org/10.1007/s10562-016-1909-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1909-0