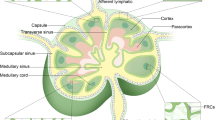
Abstract
Our understanding of the role of lymph nodes (LN) in the metastasization process (MET) is marginal. Positive LNs (pLN) are the most important prognostic factor and lymph node dissection (LND) is still standard practice in primary treatment. However, up to now, there is almost no evidence that elective LND has a survival benefit. Based on many clinical and experimental findings, we propose that tumor foci in regional LN are incapable of metastasization and can therefore not infiltrate further LN and organs. Available data demonstrate a very early infiltration of MET capable tumor cells from the primary tumor into regional LN, and thereafter an increased probability of subsequent LN infiltrations. Disparate growth rates of the first versus subsequent infiltrating tumors as well as the asymptotic growth and prognosis of large tumor foci in LN explain many clinical observations for solid tumors. The consequence of the hypothesis “pLN do not metastasize” would impact clinical treatment and research and contribute to understanding the mounting evidence against LND.





Similar content being viewed by others
Abbreviations
- BC:
-
Breast cancer
- LN:
-
Lymph node(s)
- pLN:
-
Positive lymph node(s)
- LND:
-
Lymph node dissection
- SLN:
-
Sentinel lymph node
- MET:
-
(distant) metastasization
- PT:
-
Primary tumor
- TC(D):
-
Tumor cell (dissemination)
- VD(T):
-
Volume doubling (time)
- pNITC :
-
Isolated TC in LN <0.2 mm
- pNmicro :
-
Micrometastasis in LN (0.2–2 mm)
- pN:
-
Pathological classification of regional lymph node
References
Veronesi, U., Marubini, E., Mariani, L., Valagussa, P., & Zucali, R. (1999). The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. European Journal of Cancer, 35(9), 1320–1325.
Fisher, B., Jeong, J. H., Anderson, S., Bryant, J., Fisher, E. R., & Wolmark, N. (2002). Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. The New England Journal of Medicine, 347(8), 567–575. doi:10.1056/NEJMoa020128.
Morton, D. L., Thompson, J. F., Cochran, A. J., Mozzillo, N., Elashoff, R., Essner, R., et al. (2006). Sentinel-node biopsy or nodal observation in melanoma. The New England Journal of Medicine, 355(13), 1307–1317. doi:10.1056/NEJMoa060992.
Hartgrink, H. H., van de Velde, C. J., Putter, H., Bonenkamp, J. J., Klein Kranenbarg, E., Songun, I., et al. (2004). Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. Journal of Clinical Oncology, 22(11), 2069–2077. doi:10.1200/JCO.2004.08.026.
Rouffet, F., Hay, J. M., Vacher, B., Fingerhut, A., Elhadad, A., Flamant, Y., et al. (1994). Curative resection for left colonic carcinoma: hemicolectomy vs. segmental colectomy. A prospective, controlled, multicenter trial. French Association for Surgical Research. Diseases of the Colon and Rectum, 37(7), 651–659.
Kitchener, H., Swart, A. M., Qian, Q., Amos, C., & Parmar, M. K. (2009). Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. The Lancet, 373(9658), 125–136. doi:10.1016/S0140-6736(08)61766-3.
Panici, P. B., Maggioni, A., Hacker, N., Landoni, F., Ackermann, S., Campagnutta, E., et al. (2005). Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. Journal of the National Cancer Institute, 97(8), 560–566. doi:10.1093/jnci/dji102.
Veronesi, U., Viale, G., Paganelli, G., Zurrida, S., Luini, A., Galimberti, V., et al. (2010). Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Annals of Surgery, 251(4), 595–600. doi:10.1097/SLA.0b013e3181c0e92a.
Giuliano, A. E., Hunt, K. K., Ballman, K. V., Beitsch, P. D., Whitworth, P. W., Blumencranz, P. W., et al. (2011). Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. Journal of the American Medical Association, 305(6), 569–575. doi:10.1001/jama.2011.90.
Cady, B. (2007). Regional lymph node metastases; a singular manifestation of the process of clinical metastases in cancer: contemporary animal research and clinical reports suggest unifying concepts. Annals of Surgical Oncology, 14(6), 1790–1800. doi:10.1245/s10434-006-9234-2.
Engel, J., Lebeau, A., Sauer, H., & Holzel, D. (2006). Are we wasting our time with the sentinel technique? Fifteen reasons to stop axilla dissection. The Breast, 15(3), 452–455. doi:10.1016/j.breast.2005.05.009.
Gervasoni, J. E., Jr., Sbayi, S., & Cady, B. (2007). Role of lymphadenectomy in surgical treatment of solid tumors: an update on the clinical data. Annals of Surgical Oncology, 14(9), 2443–2462. doi:10.1245/s10434-007-9360-5.
Benson, J. R., & della Rovere, G. Q. (2007). Management of the axilla in women with breast cancer. The Lancet Oncology, 8(4), 331–348. doi:10.1016/S1470-2045(07)70103-1.
Leong, S. P., Cady, B., Jablons, D. M., Garcia-Aguilar, J., Reintgen, D., Jakub, J., et al. (2006). Clinical patterns of metastasis. Cancer and Metastasis Reviews, 25(2), 221–232. doi:10.1007/s10555-006-8502-8.
Sobin, L., Gospodarowicz, M., Wittekind, C. (ed) (2009). UICC: TNM classification of malignant tumors. 7th ed. New York: Wiley-Blackwell.
Munich Cancer Registry http://www.Tumorregister-muenchen.De/facts/specific_analysis.Php.
Carter, C. L., Allen, C., & Henson, D. E. (1989). Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer, 63(1), 181–187.
Michaelson, J. S., Silverstein, M., Wyatt, J., Weber, G., Moore, R., Halpern, E., et al. (2002). Predicting the survival of patients with breast carcinoma using tumor size. Cancer, 95(4), 713–723. doi:10.1002/cncr.10742.
Nguyen, D. X., Bos, P. D., & Massague, J. (2009). Metastasis: from dissemination to organ-specific colonization. Nature Reviews. Cancer, 9(4), 274–284. doi:10.1038/nrc2622.
Ulmer, A., Fischer, J. R., Schanz, S., Sotlar, K., Breuninger, H., Dietz, K., et al. (2005). Detection of melanoma cells displaying multiple genomic changes in histopathologically negative sentinel lymph nodes. Clinical Cancer Research, 11(15), 5425–5432. doi:10.1158/1078-0432.CCR-04-1995.
Viale, G., Dell’Orto, P., Biasi, M. O., Stufano, V., De Brito Lima, L. N., Paganelli, G., et al. (2008). Comparative evaluation of an extensive histopathologic examination and a real-time reverse-transcription–polymerase chain reaction assay for mammaglobin and cytokeratin 19 on axillary sentinel lymph nodes of breast carcinoma patients. Annals of Surgery, 247(1), 136–142. doi:10.1097/SLA.0b013e318157d22b.
Cserni, G. (2008). Axillary sentinel lymph node micrometastases with extracapsular extension: a distinct pattern of breast cancer metastasis? Journal of Clinical Pathology, 61(1), 115–118. doi:10.1136/jcp.2007.047357.
van Deurzen, C. H., van Hillegersberg, R., Hobbelink, M. G., Seldenrijk, C. A., Koelemij, R., & van Diest, P. J. (2007). Predictive value of tumor load in breast cancer sentinel lymph nodes for second echelon lymph node metastases. Cellular Oncology, 29(6), 497–505.
Langer, I., Guller, U., Berclaz, G., Koechli, O. R., Schaer, G., Fehr, M. K., et al. (2007). Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Annals of Surgery, 245(3), 452–461. doi:10.1097/01.sla.0000245472.47748.ec.
Gobardhan, P. D., Elias, S. G., Madsen, E. V., Bongers, V., Ruitenberg, H. J., Perre, C. I., et al. (2009). Prognostic value of micrometastases in sentinel lymph nodes of patients with breast carcinoma: a cohort study. Annals of Oncology, 20(1), 41–48. doi:10.1093/annonc/mdn535.
Reed, J., Rosman, M., Verbanac, K. M., Mannie, A., Cheng, Z., & Tafra, L. (2009). Prognostic implications of isolated tumor cells and micrometastases in sentinel nodes of patients with invasive breast cancer: 10-year analysis of patients enrolled in the prospective East Carolina University/Anne Arundel Medical Center Sentinel Node Multicenter Study. Journal of the American College of Surgery, 208(3), 333–340. doi:10.1016/j.jamcollsurg.2008.10.036.
Colleoni, M., Rotmensz, N., Peruzzotti, G., Maisonneuve, P., Mazzarol, G., Pruneri, G., et al. (2005). Size of breast cancer metastases in axillary lymph nodes: clinical relevance of minimal lymph node involvement. Journal of Clinical Oncology, 23(7), 1379–1389. doi:10.1200/JCO.2005.07.094.
Straver, M. E., Meijnen, P., van Tienhoven, G., van de Velde, C. J., Mansel, R. E., Bogaerts, J., et al. (2010). Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Annals of Surgical Oncology, 17(7), 1854–1861. doi:10.1245/s10434-010-0945-z.
van Diest, P. J. (1999). Histopathological workup of sentinel lymph nodes: how much is enough? Journal of Clinical Pathology, 52(12), 871–873.
Weaver, D. L., Krag, D. N., Manna, E. A., Ashikaga, T., Waters, B. L., Harlow, S. P., et al. (2006). Detection of occult sentinel lymph node micrometastases by immunohistochemistry in breast cancer. An NSABP protocol B-32 quality assurance study. Cancer, 107(4), 661–667.
Peer, P. G., van Dijck, J. A., Hendriks, J. H., Holland, R., & Verbeek, A. L. (1993). Age-dependent growth rate of primary breast cancer. Cancer, 71(11), 3547–3551.
Tilanus-Linthorst, M. M., Kriege, M., Boetes, C., Hop, W. C., Obdeijn, I. M., Oosterwijk, J. C., et al. (2005). Hereditary breast cancer growth rates and its impact on screening policy. European Journal of Cancer, 41(11), 1610–1617. doi:10.1016/j.ejca.2005.02.034.
Tanis, P. J., Nieweg, O. E., Valdes Olmos, R. A., & Kroon, B. B. (2001). Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. Journal of the American College of Surgery, 192(3), 399–409.
Borgstein, P. J., Meijer, S., Pijpers, R. J., & van Diest, P. J. (2000). Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Annals of Surgery, 232(1), 81–89.
Treseler, P. A., & Tauchi, P. S. (2000). Pathologic analysis of the sentinel lymph node. The Surgical Clinics of North America, 80(6), 1695–1719.
Fisher, B., & Fisher, E. R. (1966). The interrelationship of hematogenous and lymphatic tumor cell dissemination. Surgery, Gynecology & Obstetrics, 122(4), 791–798.
Cady, B. (1984). Lymph node metastases. Indicators, but not governors of survival. Archives of Surgery, 119(9), 1067–1072.
Fisher, B., & Fisher, E. R. (1966). Transmigration of lymph nodes by tumor cells. Science, 152(727), 1397–1398.
Nakagawa, T., Martinez, S. R., Goto, Y., Koyanagi, K., Kitago, M., Shingai, T., et al. (2007). Detection of circulating tumor cells in early-stage breast cancer metastasis to axillary lymph nodes. Clinical Cancer Research, 13(14), 4105–4110. doi:10.1158/1078-0432.CCR-07-0419.
Riethdorf, S., & Pantel, K. (2010). Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Annals of the New York Academy of Sciences, 1210, 66–77. doi:10.1111/j.1749-6632.2010.05779.x.
Sinha, P. S., Thrush, S., Bendall, S., & Bates, T. (2002). Does radical surgery to the axilla give a survival advantage in more severe breast cancer? European Journal of Cancer, 38(11), 1474–1477.
Weinberg, R. (2007). The biology of cancer. New York: Garland Science.
Naumov, G. N., Folkman, J., Straume, O., & Akslen, L. A. (2008). Tumor–vascular interactions and tumor dormancy. APMIS, 116(7–8), 569–585. doi:10.1111/j.1600-0463.2008.01213.x.
Ding, L., Ellis, M. J., Li, S., Larson, D. E., Chen, K., Wallis, J. W., et al. (2010). Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature, 464(7291), 999–1005. doi:10.1038/nature08989.
Yachida, S., Jones, S., Bozic, I., Antal, T., Leary, R., Fu, B., et al. (2010). Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature, 467(7319), 1114–1117. doi:10.1038/nature09515.
Becker, T. E., Ellsworth, R. E., Deyarmin, B., Patney, H. L., Jordan, R. M., Hooke, J. A., et al. (2008). The genomic heritage of lymph node metastases: implications for clinical management of patients with breast cancer. Annals of Surgical Oncology, 15(4), 1056–1063. doi:10.1245/s10434-008-9815-3.
Chen, S. L., Hoehne, F. M., & Giuliano, A. E. (2007). The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Annals of Surgical Oncology, 14(12), 3378–3384. doi:10.1245/s10434-007-9513-6.
Cox, C. E., Kiluk, J. V., Riker, A. I., Cox, J. M., Allred, N., Ramos, D. C., et al. (2008). Significance of sentinel lymph node micrometastases in human breast cancer. Journal of the American College of Surgery, 206(2), 261–268. doi:10.1016/j.jamcollsurg.2007.08.024.
Andersson, Y., Frisell, J., Sylvan, M., de Boniface, J., & Bergkvist, L. (2010). Breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Journal of Clinical Oncology, 28(17), 2868–2873. doi:10.1200/JCO.2009.24.5001.
Belt, E. J., van Stijn, M. F., Bril, H., de Lange-de Klerk, E. S., Meijer, G. A., Meijer, S., et al. Lymph node negative colorectal cancers with isolated tumor deposits should be classified and treated as stage iii. Annals of Surgical and Oncology, doi:10.1245/s10434-010-1152-7.
Pugliese, M. S., Beatty, J. D., Tickman, R. J., Allison, K. H., Atwood, M. K., Szymonifka, J., et al. (2009). Impact and outcomes of routine microstaging of sentinel lymph nodes in breast cancer: significance of the pN0(i+) and pN1mi categories. Annals of Surgical Oncology, 16(1), 113–120. doi:10.1245/s10434-008-0121-x.
de Boer, M., van Deurzen, C. H., van Dijck, J. A., Borm, G. F., van Diest, P. J., Adang, E. M., et al. (2009). Micrometastases or isolated tumor cells and the outcome of breast cancer. The New England Journal of Medicine, 361(7), 653–663. doi:10.1056/NEJMoa0904832.
Weaver, D. L., Ashikaga, T., Krag, D. N., Skelly, J. M., Anderson, S. J., Harlow, S. P., et al. (2011). Effect of occult metastases on survival in node-negative breast cancer. The New England Journal of Medicine. doi:10.1056/NEJMoa1008108.
Colpaert, C., Vermeulen, P., Jeuris, W., van Beest, P., Goovaerts, G., Weyler, J., et al. (2001). Early distant relapse in “node-negative” breast cancer patients is not predicted by occult axillary lymph node metastases, but by the features of the primary tumour. The Journal of Pathology, 193(4), 442–449. doi:10.1002/path.829.
Hölzel, D., Eckel, R., Emeny, R., & Engel, J. (2010). Distant metastases do not metastasize. Cancer and Metastasis Reviews, 29, 737–750. doi:10.1007/s10555-010-9260-1.
Suzuki, M., Mose, E. S., Montel, V., & Tarin, D. (2006). Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. American Journal of Pathology, 169(2), 673–681.
Louis-Sylvestre, C., Clough, K., Asselain, B., Vilcoq, J. R., Salmon, R. J., Campana, F., et al. (2004). Axillary treatment in conservative management of operable breast cancer: dissection or radiotherapy? Results of a randomized study with 15 years of follow-up. Journal of Clinical Oncology, 22(1), 97–101. doi:10.1200/JCO.2004.12.108.
van Wely, B. J., Teerenstra, S., Schinagl, D. A., Aufenacker, T. J., de Wilt, J. H., & Strobbe, L. J. Systematic review of the effect of external beam radiation therapy to the breast on axillary recurrence after negative sentinel lymph node biopsy. British Journal of Surgery, doi:10.1002/bjs.7360.
Straver, M. E., Meijnen, P., van Tienhoven, G., van de Velde, C. J., Mansel, R. E., Bogaerts, J., et al. (2010). Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. Journal of Clinical Oncology, 28(5), 731–737. doi:10.1200/JCO.2008.21.7554.
Gill, P. G., Birrell, S. N., Luke, C. G., & Roder, D. M. (2002). Tumour location and prognostic characteristics as determinants of survival of women with invasive breast cancer: South Australian hospital-based cancer registries, 1987–1998. The Breast, 11(3), 221–227. doi:10.1054/brst.2001.0400.
Janni, W., Rack, B., Sommer, H., Schmidt, M., Strobl, B., Rjosk, D., et al. (2003). Intra-mammary tumor location does not influence prognosis but influences the prevalence of axillary lymph-node metastases. Journal of Cancer Research and Clinical Oncology, 129(9), 503–510. doi:10.1007/s00432-003-0465-3.
Jayasinghe, U. W., & Boyages, J. (2009). Tumour location is not an independent prognostic factor for survival following a diagnosis of breast cancer. The Breast, 18(1), 41–46. doi:10.1016/j.breast.2008.10.004.
Hölzel, D., Emeny, R., & Engel, J. (2011). True local recurrences do not metastasize. Cancer and Metastasis Reviews, 30, 161–176. doi:10.1007/s10555-011-9275-2.
van de Vijver, M. J., He, Y. D., van’t Veer, L. J., Dai, H., Hart, A. A., Voskuil, D. W., et al. (2002). A gene-expression signature as a predictor of survival in breast cancer. The New England Journal of Medicine, 347(25), 1999–2009. doi:10.1056/NEJMoa021967.
van’t Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415(6871), 530–536. doi:10.1038/415530a.
Wang, Y., Klijn, J. G., Zhang, Y., Sieuwerts, A. M., Look, M. P., Yang, F., et al. (2005). Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. The Lancet, 365(9460), 671–679. doi:10.1016/S0140-6736(05)17947-1.
Minn, A. J., Gupta, G. P., Siegel, P. M., Bos, P. D., Shu, W., Giri, D. D., et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature, 436(7050), 518–524. doi:10.1038/nature03799.
Liu, R., Wang, X., Chen, G. Y., Dalerba, P., Gurney, A., Hoey, T., et al. (2007). The prognostic role of a gene signature from tumorigenic breast-cancer cells. The New England Journal of Medicine, 356(3), 217–226. doi:10.1056/NEJMoa063994.
Bos, P. D., Zhang, X. H., Nadal, C., Shu, W., Gomis, R. R., Nguyen, D. X., et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature, 459(7249), 1005–1009. doi:10.1038/nature08021.
Heyn, C., Ronald, J. A., Ramadan, S. S., Snir, J. A., Barry, A. M., MacKenzie, L. T., et al. (2006). In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magnetic Resonance in Medicine, 56(5), 1001–1010. doi:10.1002/mrm.21029.
Goguen, L. A., Chapuy, C. I., Sher, D. J., Israel, D. A., Blinder, R. A., Norris, C. M., et al. Utilizing computed tomography as a road map for designing selective and superselective neck dissection after chemoradiotherapy. Otolaryngology—Head and Neck Surgery, 143(3), 367–374, doi:S0194-5998(10)00399-2 [pii] 10.1016/j.otohns.2010.04.020.
Koch, W. M., Ridge, J. A., Forastiere, A., & Manola, J. (2009). Comparison of clinical and pathological staging in head and neck squamous cell carcinoma: results from intergroup study ECOG 4393/RTOG 9614. Archives of Otolaryngology—Head & Neck Surgery, 135(9), 851–858. doi:10.1001/archoto.2009.123.
Talmadge, J. E., Wolman, S. R., & Fidler, I. J. (1982). Evidence for the clonal origin of spontaneous metastases. Science, 217(4557), 361–363.
Luebeck, E. G. (2010). Cancer: genomic evolution of metastasis. Nature, 467(7319), 1053–1055. doi:10.1038/4671053a.
Campbell, P. J., Yachida, S., Mudie, L. J., Stephens, P. J., Pleasance, E. D., Stebbings, L. A., et al. (2010). The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature, 467(7319), 1109–1113. doi:10.1038/nature09460.
Pleasance, E. D., Cheetham, R. K., Stephens, P. J., McBride, D. J., Humphray, S. J., Greenman, C. D., et al. (2010). A comprehensive catalogue of somatic mutations from a human cancer genome. Nature, 463(7278), 191–196. doi:10.1038/nature08658.
Navin, N., Kendall, J., Troge, J., Andrews, P., Rodgers, L., McIndoo, J., et al. (2011). Tumour evolution inferred by single-cell sequencing. Nature. doi:10.1038/nature09807.
Stratton, M. R., Campbell, P. J., & Futreal, P. A. (2009). The cancer genome. Nature, 458(7239), 719–724. doi:10.1038/nature07943.
Feinstein, A. R., Sosin, D. M., & Wells, C. K. (1985). The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. The New England Journal of Medicine, 312(25), 1604–1608.
George, S., Primrose, J., Talbot, R., Smith, J., Mullee, M., Bailey, D., et al. (2006). Will Rogers revisited: prospective observational study of survival of 3592 patients with colorectal cancer according to number of nodes examined by pathologists. British Journal of Cancer, 95(7), 841–847. doi:10.1038/sj.bjc.6603352.
Butler, T. P., & Gullino, P. M. (1975). Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Research, 35(3), 512–516.
Glaves, D., Huben, R. P., & Weiss, L. (1988). Haematogenous dissemination of cells from human renal adenocarcinomas. British Journal of Cancer, 57(1), 32–35.
Acknowledgments
We thank the many doctors and clinicians who cooperate within the complex network of the Munich Cancer Registry (MCR), despite the back-breaking medical bureaucracy required for daily health care. Especially, we thank our co-workers in the MCR who handle hundreds of thousands of findings and treatment reports and compile them into valid courses of disease. We are also indebted to I Bauerfeind, HP Bruch, J Haier, R Holland, M Hölzel, CA Klein, U Löhrs, D Meyer, K Pantel, G Riethmüller, H-J Sauer, U Schumacher, J. Werner, and F Winkler for insightful information and discussions, especially concerning contrary positions, because dissent rather than consensus is a prime motivation for innovations.
Conflicts of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engel, J., Emeny, R.T. & Hölzel, D. Positive lymph nodes do not metastasize. Cancer Metastasis Rev 31, 235–246 (2012). https://doi.org/10.1007/s10555-011-9343-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9343-7