Abstract
Cardiac magnetic resonance (CMR) has changed the management of suspected viral myocarditis by providing a ‘positive’ diagnostic test and has lead to new insights into myocardial involvement in systemic inflammatory conditions. In this review we analyse the use of CMR tissue characterisation techniques across the available studies including T2 weighted imaging, early gadolinium enhancement, late gadolinium enhancement, Lake Louise Criteria, T2 mapping, T1 mapping and extracellular volume assessment. We also discuss the use of multiparametric CMR in acute cardiac transplant rejection and a variety of inflammatory conditions such as sarcoidosis, systemic lupus erythrematous, rheumatoid arthritis and systemic sclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organisation defines myocarditis as an inflammatory disease of the myocardium diagnosed by established histological, immunological and immunohistochemical criteria [1]. Myocardial involvement in presumed systemic viral infection is the most common aetiology, although it can result from a wide spectrum of infectious pathogens and non-infectious causes including systemic inflammatory conditions and toxins [2, 3].
Clinical presentation is often non-specific and heterogeneous, ranging from symptoms of chest pain, dyspnoea, fatigue or palpitations to brady- and tachy-arrhythmias, cardiogenic shock and sudden death [2]. Peripheral markers of inflammation (e.g. c-reactive protein) and myocardial injury (e.g. troponin) lack sensitivity and specificity, and viral serology is unhelpful [4–6]. Invasive endomyocardial biopsy (EMB) is recommended in specific scenarios, such as “New-onset heart failure of 2 weeks duration associated with a normal-sized or dilated left ventricle and hemodynamic compromise (IB)” and “New-onset heart failure of 2 weeks to 3 months duration associated with a dilated left ventricle and new ventricular arrhythmias, second- or third-degree heart block, or failure to respond to usual care within 1 to 2 weeks (1B)”, [7] however, it is associated with a risk of complications (1–2%) and due to sampling error, transiency of myocardial injury and variation in histology interpretation, it also lacks accuracy [2, 8–12]. In most centres it is rarely performed. As a result of these factors, the diagnosis of myocarditis is challenging, and has traditionally been made after other cardiac diseases have been excluded [2].
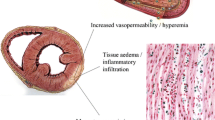
Over the past decade, cardiac magnetic resonance (CMR) has changed this paradigm. The unique ability of multiparametric CMR to characterise myocardial tissue, and thus potentially detect the myocardial oedema, increased blood flow and capillary leakage, necrosis and subsequent fibrosis that occurs in myocarditis, coupled with the ability of CMR to detect subtle regional or global contractile dysfunction, means that CMR is now often able to provide a positive diagnosis of myocarditis. Indeed, CMR has provided pathophysiological insight into the nature of the myocardial injury in myocarditis.
This review will describe the diagnostic utility of CMR parameters across a range of myocarditic aetiologies. In this context, it is important to recognise that the evaluation of CMR, or indeed any diagnostic test, in myocarditis is limited by the lack of a good reference standard. Histological validation is challenging and imperfect, as described. As a result, many studies use a clinical diagnosis of myocarditis as the reference, however this is inherently limited. In addition, heterogeneous study designs and patient populations (e.g. acute versus chronic myocardial inflammation, definition of control groups), and the nature of CMR (differing magnetic field strengths, imaging sequences, measured parameters) makes comparisons between studies difficult.
Idiopathic (presumed viral) myocarditis
In North America and Europe, myocardial involvement in presumed systemic viral infection remains the most common aetiology of myocarditis [2, 13–18].
T2 weighted imaging
T2 relaxation is directly proportional to tissue water content, and T2 weighted (T2w) imaging has been proposed to detect myocardial oedema [3, 19, 20].
Table 1 summarises studies that have evaluated the diagnostic performance of T2w imaging, including the sequences employed, the populations studied and the reference standards [21–35]. Most studies analyse T2w images using an oedema ratio (ER), defined as the ratio of myocardial to skeletal muscle signal intensity (SI), with values above a set value considered pathological. However, the threshold varies across studies (1.8–2.2), is usually determined retrospectively and the technique is hampered by potential coexistence of myositis and a lack of skeletal muscle in the field of view [21, 35]. A minority of studies have used qualitative assessment, although a lack of ‘healthy’ myocardium for comparison in the context of global myocarditis is a limitation [35].
The pooled weighted sensitivity, specificity and diagnostic accuracy of T2w for diagnosing acute myocarditis are 63, 76 and 68% respectively.
In the largest study (104 patients), in which a clinical diagnosis of myocarditis was used as the reference standard, Radunski et al. reported a modest diagnostic accuracy (70%) [31]. Median interval between symptom onset and scan was 2 weeks, however the interquartile range was up to 7 weeks, by which time patients may have been in the convalescent stage. Indeed, the effect of delayed scan timing on T2w imaging sensitivity was investigated by Monney et al [36] and Hinojar et al [34], who found a higher prevalence of abnormal signal on T2w images when scanning within 2 weeks of symptom onset (81 and 56% respectively) compared to scanning performed later (11% at 39 days [36] and 12% at 6 months [34]). Other studies comparing acute and convalescent imaging have also shown that high T2 signal is a transient feature of inflammatory response [36–40]. In addition, abnormalities detectable on T2w imaging appear to vary according to clinical presentation, with a higher prevalence in the context of infarction-like symptoms (81% sensitivity) and much lower in the setting of heart failure or arrhythmias (sensitivity 28 and 27% respectively) [41].
Early gadolinium enhancement
Early gadolinium enhancement (EGE) exploits the phenomenon of regional vasodilatation, increased blood flow and capillary leakage present in an inflammatory process which results in increased contrast retention in the early washout period [3].
Table 2 summarises studies that have evaluated the diagnostic performance of EGE imaging [3, 21–27, 29, 31–33, 35, 42]. Analysis of EGE images is performed using Myocardial Signal Enhancement, defined as myocardial SI post-contrast minus myocardial SI pre- contrast divided by myocardial SI pre- contrast, with values above 45–56% considered pathological [21, 29, 31], or more commonly, the global relative enhancement (gRE),[42] which is calculated as myocardial signal enhancement divided by skeletal muscle signal enhancement. Most studies use a gRE value of 4.0 as the threshold between healthy and abnormal myocardium [22–27, 32]. Such analyses have similar disadvantages to the ER.
The pooled weighted sensitivity, specificity and diagnostic accuracy of EGE for diagnosing acute myocarditis are 66, 70 and 67% respectively, with a wide range of diagnostic performances reported for both myocardial signal enhancement and gRE analysis techniques. Interestingly, Bohnen et al. found no statistical difference in gRE between heart failure patients with histologically confirmed inflammation and those without [43].
Friedrich et al [42, 44] found the pattern of signal enhancement was localised within first week but subsequently became more diffuse. By day 14, gRE values stopped being significantly higher in the myocarditis group compared to the control group. Studies comparing EGE in acute and convalescent phases show a significant drop in gRE, from 4.1–8.5 during acute presentation to 2.4–4.4 at follow up (performed 3–28 months later) [37–40].
Late gadolinium enhancement
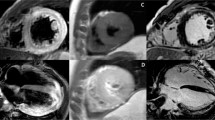
Late gadolinium enhancement (LGE) was originally thought to demonstrate irreversible myocardial injury only, however several studies have demonstrated a temporal change in the extent of LGE in myocarditis, with LGE volume seen to decrease significantly over time (follow up scans performed between 1 and 18 months) [3, 36–38, 40, 45]. Histological correlation has shown LGE is associated with active inflammation, with the extent of LGE corresponding to the severity of the inflammatory histopathological findings [45, 46]. It is likely that LGE in acute myocarditis represents both reversible and irreversible myocardial injury, but in the chronic phase represents residual focal fibrosis. See Fig. 1a for a representative example.
Patient with acute viral myocarditis. a Late enhancement imaging. Epicardial and mid-wall late enhancement (green arrows) in mid anterolateral and apical lateral segments. b T1 mapping, MOLLI sequence. Elevated T1 values in mid-wall and epicardial portion of basal—mid anterolateral and apical lateral segments (green arrows; T1 values in anterolateral wall: 1152 ms, T1 values in basal inferoseptum: 1031 ms). c T2 mapping, T2-prepared SFFP sequence. Elevated T2 values in epicardial portion of mid anterolateral and apical lateral segments (green arrows; T2 values in mid anterolateral segment: 66 ms, T2 values in basal inferoseptum: 47 ms)
Table 3 summarises studies that have evaluated the diagnostic performance of LGE imaging [22–27, 29–35, 45, 47]. The pooled weighted sensitivity, specificity and diagnostic accuracy of LGE for diagnosing acute myocarditis are 65, 95 and 75% respectively. The prevalence of LGE varies considerably across studies (27–95%), likely reflecting the heterogeneity of the populations studied and the timing of CMR [22–27, 29–36, 40, 45, 47–51].
While a number of studies have shown that LGE can involve any region of the LV (or the right ventricle), Mahrholdt et al.[46] in seminal work, showed LGE is most commonly located in the lateral LV, typically originating from the epicardial quartile of the LV wall. Transmural lateral wall LGE, possibly reflecting very florid disease, is reported in a minority [22, 23, 26, 27, 30, 33, 36, 40, 45, 47, 49–53]. The distribution of LGE may be associated with the infecting pathogen, with parvovirus B19 found to be association with sub-epicardial lateral wall LGE, whereas human herpes virus 6 is associated with mid wall septal LGE [45].
LGE imaging requires the presence of ‘normal’ myocardium as a reference, thus may not be sensitive to diffuse disease [54].
Lake Louise criteria
In an effort to increase the diagnostic performance of CMR, the three tissue characterisation techniques discussed above (T2w imaging, EGE and LGE) were combined to form the Lake Louise Criteria (LLC). In the setting of clinically suspected myocarditis, abnormal findings on two of the three techniques were determined to be consistent with myocardial inflammation [3].
Table 4 summarises studies that have evaluated the diagnostic performance of the LLC [22, 23, 25–27, 31–33, 35]. The pooled weighted sensitivity, specificity and diagnostic accuracy of the LLC for diagnosing acute myocarditis are 80, 87 and 83% respectively, and as such the LLC demonstrate a better overall diagnostic performance than any of the individual CMR parameters. Similar to T2w imaging, LLC appears to have better diagnostic performance in “infarct-like” presentation (sensitivity of 80%) compared to heart failure or arrhythmias (sensitivity 57 and 40% respectively) [41].
Parametric mapping
In recent years, parametric mapping, which allows direct quantification of myocardial tissue magnetic parameters (primarily T1 and T2) has been increasingly applied in myocarditis. (Similar to T2, T1 relaxation times are sensitive to changes in myocardial water content and have been proposed to detect myocardial oedema). As well as being associated with potentially less observer variability, less artefact and allowing global myocardial assessment, native T1 and T2 mapping offer the significant advantage of not requiring contrast agent administration. See Fig. 1b, c for representative examples.
Table 5 summarises the studies that have evaluated the diagnostic performance of T2 and T1 mapping. The pooled weighted sensitivity, specificity and diagnostic accuracy of T2 mapping for diagnosing acute myocarditis are 70, 91 and 79% respectively [31, 35, 43, 51, 55]. The pooled weighted sensitivity, specificity and diagnostic accuracy of T1 mapping are 82, 91 and 86% [30–32, 34, 35]. Thus the diagnostic performance of T2 mapping is comparable to that of the LLC, while the performance of T1 mapping may be superior.
Luetkens et al. compared the diagnostic performance of CMR parameters in two studies, albeit in relatively small populations (24 and 34 patients with myocarditis respectively), and demonstrated similar findings. In the first study, which did not include T2 mapping [32], native T1 mapping was associated with the highest diagnostic performance (area under the curve, AUC 0.94), followed by LGE (AUC 0.9), LLC (AUC 0.86), ER (AUC 0.79) and gRE (AUC 0.63). In the second study, which included T2 mapping,[35], the performance of native T1 mapping (AUC 0.92–0.95) and T2 mapping (AUC 0.92) was very similar. Combining T1 mapping with LGE (diagnostic accuracy 91–96%) [30, 32, 34, 35] or T2 mapping and LGE (diagnostic accuracy 96%) [35] may improve diagnostic performance further.
Nevertheless, there are a number of areas which require further investigation. Only one study has compared T1 and T2 mapping with histological findings in myocarditis. Relaxation time thresholds for diagnosing myocarditis have generally been determined retrospectively. T1 relaxation time diagnostic thresholds vary considerably between studies (852–1074 ms at 1.5 T). T2 relaxation time diagnostic thresholds are generally much more consistent (approximately 60 ms), however they overlap considerably with published normal ranges (up to 65 ms) [51, 56–66]. A prospective, multicentre, multivendor trial with predetermined diagnostic thresholds is required to determine the clinical diagnostic utility of mapping with quantitative analysis before this technique can enter clinical practice.
Other noteworthy findings include those of Hinojar et al. who showed elevated T1 values (compared to healthy controls) persisted for up to 4–8 months post initial presentation [34]. Bohnen et al. found no difference in T1 values in patients with heart failure and histologically confirmed inflammation compared to patients with heart failure and no evidence of inflammation on histology [43]. This may reflect the fact that native T1 is determined by a number of factors other than inflammation (e.g. fibrosis).
Only three studies have examined the diagnostic utility of ECV in myocarditis, with varying results (Table 5) [31, 32, 35].
Acute cardiac allograft rejection
Acute cardiac allograft rejection (ACAR) is a leading cause of death in the first year post heart transplant, however clinical features are unreliable. Routine screening is therefore performed in order to detect ACAR and hence augment immunosuppressive therapy, at an earlier stage, with the aim of preventing progression to more severe disease [67, 68]. Histological analysis of myocardial tissue obtained at EMB remains the gold standard for ACAR surveillance however it is associated with a number of limitations. CMR is a potentially attractive screening modality.
In one of the largest human studies, which included 68 patients undergoing 123 CMR scans, T2 relaxation time was significantly higher in grade 2 ACAR (57 ± 5 ms) compared with grade 0 or 1 (50 ± 5 ms and 51 ± 8 ms, respectively); and in grade 3 (65 ± 8 ms) compared with grade 2 [69]. A T2 relaxation time of ≥56 ms, determined retrospectively, had a high NPV (97%) for detecting significant ACAR (≥grade 2). More recently in a study of approximately 50 patients undergoing 68 CMR scans, Usman et al. found myocardial T2 was significantly higher in the ACAR group (including 4 cases of >grade 2R ACAR, two cases of antibody-mediated rejection and two cases where ACAR treatment was started on the basis of high clinical suspicion alone) compared to the non-ACAR group [70]. A T2 of 56.4 ms yielded a sensitivity and specificity of 86.5 and 94.6% respectively. However, both studies specifically selected patients who were known to have/suspected of having ACAR. Furthermore, patients were a scanned at a substantial time post-transplant (Marie et al. up to 6 years, Usman et al. up to 2 years), thus missing the window in which early detection of ACAR is thought to be most useful, indeed the benefit of routine screening later than one year post-transplant is subject to debate.
In a study of 22 patients undergoing 88 CMR scans over the first 5 months post-transplant, Miller et al. found myocardial T1 and T2 were not significantly higher in grade 2R ACAR compared to grades 0R-1R [71]. However the study did demonstrate significant improvements in markers of LV structure and contractility, native T1, T2 and ECV and microvascular function over the period studied, providing insight into the myocardial injury associated with transplantation, and its recovery.
It may be that CMR parameters become more useful for detecting ACAR as time from transplantation increases and the transplant-related myocardial injury subsides. The paradox however is that while non-invasive approaches to ACAR surveillance may become more discriminatory as time from transplantation increases, the benefit of the early detection of ACAR diminishes [71].
Sarcoidosis
Sarcoidosis is a multi-organ systemic inflammatory disorder characterized by the formation of non-caseating granulomas [72]. Autopsy studies suggest cardiac sarcoidosis is a major cause for sarcoid-related mortality, however pre-mortem diagnosis of cardiac sarcoid is challenging [72, 73]. Endomyocardial biopsy and clinical diagnostic criteria [74] are limited [75].
Smedema et al. [76] found LGE in all patients (n = 12) meeting clinical criteria for cardiac sarcoid, and in a further 17% who did not meet the criteria. Patel et al. [77] showed CMR identified twice as many patients (n = 21) with evidence of myocardial involvement as clinical evaluation, which included 12-lead ECG and at last one non-CMR cardiac investigation (echocardiography, radionuclide scintigraphy or cardiac catheterisation).
Regional and mural LGE distribution in cardiac sarcoid is markedly heterogeneous. LGE has been demonstrated in all LV and RV regions, albeit with some predilection to basal septal regions [76–80]. Subendocardial, mid wall, epicardial and transmural patterns have been described [76–80]. Using T2 mapping, Crouser et al. [81] found significantly higher myocardial T2 values amongst 50 consecutive patients investigated for cardiac sarcoid compared to healthy controls. T2 cut off of 59 ms achieved sensitivity of 54% and specificity of 100%.
18F-fluoro-2-deoxyglucose positron emission tomography (18F-FDG PET; a marker of active inflammation) studies have provided insight into the CMR findings [82, 83]. T2w signal and LGE have been demonstrated to correspond to regions taking up 18F-FDG, with reduced uptake following corticosteroids, indicating active inflammation. However, LGE is also found in regions without 18F-FDG uptake, indicating fibrotic lesions. Thus T2w signal may reflect active inflammation, whereas LGE may reflect either active inflammation or fibrosis.
The presence of LGE is associated with a higher rate of sudden cardiac death (SCD) and ventricular tachyarrhythmia, although this requires further assessment in larger studies [77–79, 84].
Systemic lupus erythematous
Systemic lupus erythematous (SLE) is a multisystem inflammatory disorder [85].
Cardiovascular involvement represents a significant cause of morbidity and mortality [86]. SLE associated myocarditis was shown to shorten the survival and is more common amongst patients with higher disease activity [87]. There is also a discrepancy between the number of myocarditis cases detected on autopsy and clinical diagnoses, suggesting common subclinical cardiac involvement [88, 89]. There is considerable interest in the accurate detection of myocardial involvement in SLE, and other rheumatological conditions, as it may potentially guide therapy aimed at reducing adverse cardiovascular outcomes.
A small study by Singh et al. [90] showed that T2 relaxation times were higher in six patients with active SLE compared to five with lower disease activity and five healthy controls (T2 values of 82, 64 and 65 ms respectively). Similarly, Abdel-Aty et al. [88] showed that both ER and gRE were significantly higher in patients with active disease, both correlated to disease activity and ER significantly decreased with clinical improvement.
Mavrogeni et al. [89] compared a group of twenty-five patients with active SLE and suspected cardiac involvement with fifty patients suspected of having viral myocarditis showing no statistical difference in ER and EGE, potentially suggesting similar myocardial pathological processes in both conditions.
Puntmann et al. [91] showed that T1 and ECV values were significantly higher among thirty-three SLE patients in clinical remission compared to twenty-one healthy controls (T1 1152 ± 46 vs. 1056 ± 27 ms, p < 0.001; ECV 30 ± 6% versus 26 ± 5%, p = 0.007). A challenge for the CMR community is to decipher whether such findings represent active inflammation or chronic fibrosis, or indeed both. The authors did not perform T2 mapping, however, ER did not differ between groups, potentially suggesting the T1 and ECV findings may represent fibrosis. Conversely, Zhang et al. [92] demonstrated higher T2 values in twenty-four SLE patients with low disease activity compared to twelve healthy controls (58.2 ± 5.6 vs. 52.8 ± 4.4 ms), which the authors suggested may represent ongoing myocardial inflammation.
LGE may be less prevalent in SLE. Zhang et al. [92] observed no late enhancement amongst twenty-four SLE patients while Mavrogeni et al. [89] found significantly less LGE amongst patients with active SLE compared to viral myocarditis (LGE volume 3.5 ± 5.5 vs. 8 ± 4.4%, p < 0.001), possibly reflecting a more diffuse nature of myocardial involvement.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease [93]. Cardiovascular involvement is common, manifesting as coronary artery disease, myocardial inflammation and fibrosis, and is responsible for 40–80% of premature deaths [94–97].
Kobayashi et al. [98] examined eighteen RA patients without a previous history of cardiovascular conditions, finding LGE in almost 40% of patients, with a mostly non-ischaemic distribution. The presence of LGE was correlated to higher disease activity scores (DAS28 4.77 vs. 3.44, p = 0.011).
Mavrogeni et al. [99] used T2w imaging, EGE and LGE to compare two groups of RA patients in remission: twenty with and twenty without recent onset cardiac symptoms. 10% of patients with symptoms had evidence of myocardial infarction with a typical ischaemic LGE pattern and 65% displayed evidence of myocarditis as defined by LLC. Over three quarters of those diagnosed with myocarditis experienced an RA relapse within 6 weeks, possibly suggesting more active disease.
Ntusi et al. [100] found LGE to be present in almost half of twenty-eight examined RA patients with a mostly non-ischaemic, mid wall pattern. In addition, 5% of patients were diagnosed with silent myocardial infarction based on the presence of subendocardial LGE and confirmed by coronary angiography. There was no difference in global ER between RA patients and controls, however, RA patients had more areas of elevated ER (ER > 1.9, median 10 vs. 0% amongst controls) suggesting the presence of focal myocardial oedema. Finally, global T1 values and ECV were significantly higher in the RA group (T1 973 ± 27 vs. 961 ± 18 ms, p = 0.03; ECV 30.3 ± 3.4 vs. 27.9 ± 2%, p < 0.001). Although, in keeping with the findings in SLE, it is not clear to what extent these findings represent active inflammation or fibrosis and the magnitude of the difference in global T1, whilst statistically significant, were small.
It is clear from these CMR studies that subclinical cardiac involvement is common. CMR parameters have the potential to risk stratify and guide therapy in RA, although further work is required to define the nature of the CMR findings in RA and their accuracy and reproducibility in this population.
Systemic sclerosis
Systemic Sclerosis (SSC) is an autoimmune connective tissue disorder characterised by multi-organ fibrosis [101]. Cardiac involvement in SSC is estimated at 15–35% [101] and includes myocardial fibrosis, myocarditis, dilated cardiomyopathy, premature coronary artery disease, conduction abnormalities, valvular and pericardial disease [102]. Myocardial pathologies are often subclinical with higher prevalence on autopsy studies [103]. Overt cardiac disease is associated with poor prognosis, with a reported 70% mortality at 5 years [104].
A number of studies have evaluated LGE in SSC patients, demonstrating a prevalence of LGE of between 4 and 66% [105–114]. LGE prevalence and distribution does not seem to differ between limited and diffuse cutaneous forms of SSC [105, 106, 108, 113]. Both non-ischaemic and ischaemic patterns of LGE are described [105–112] It is not clear whether the non-ischaemic LGE represents inflammation or fibrosis. Microvascular dysfunction is a prominent feature of SSC and diffuse myocardial ischaemia evident on perfusion imaging may be part of the pathophysiological process [107, 113].
In a study by Hachulla et al. [106] fifty-two SSC patients without prior cardiac disease were assessed by multiparametric CMR. Qualitative T2w signal was increased in 12% of participants. Ntusi et al. [110] study found nineteen SCC patients to have a significantly greater extent of high gRE values compared to twenty healthy controls [110]. There was no difference between limited and diffuse cutaneous SSC [106, 110].
T1 mapping and ECV values were also shown to be higher in SSC patients without past cardiovascular involvement. In previously mentioned study by Ntusi et al. [110], SSC participants had mean T1 values of 1007 ± 29 ms and ECV of 35.4 ± 4.8% compared to T1 of 958 ± 20 ms (p < 0.001) and ECV of 27.6 ± 2.5% (p < 0.001) amongst controls. Two further studies confirmed higher ECV in SSC patients compared to healthy controls: Barison et al. [109] (30 SSC patients, ECV 30 ± 4% vs. 28 ± 4%, p = 0.03) and Thuny et al. [115] (33 SSC patients, median ECV 30%, range 28–31.9% vs. 26.8%, range 25.4–29.1%, p = 0.001).
Conclusions
By providing a ‘positive’ diagnostic test, CMR has changed the management of suspected viral myocarditis and has provided new insight into myocardial involvement in systemic inflammatory conditions. Thus CMR has opened a window for potential therapeutic targets. Parametric mapping appears to offer advantages over more conventional CMR techniques. However, multicentre, multivendor clinical trials are required to fully establish the clinical utility of CMR in myocarditis, and, in particular, quantitative mapping analysis.
References
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I (1996) Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation 93:841–842
Caforio A, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix S, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto Y, Ristic A, Schultheiss H-P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis. Eu Heart J 34:2636–2648
Friedrich M, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper L, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy J-P, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53(17):1475–1487
Mahfoud F, Gärtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, Kandolf R, Böhm M, Kindermann I (2011) Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J 32:897–903
Laurer B, Niederau C, Ku U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP (1997) Cardiac troponin t in patients with clinically suspected myocarditis. J Am Coll Cardiol 30:1354–1359
Smith SC, Ladenson JH, Mason JW, Jaffe AS (1997) Elevations of cardiac troponin i associated with myocarditis: experimental and clinical correlates. Circulation 95:163–168
Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R (2007) The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American heart association, the American college of cardiology, and the European society of cardiology. Circulation 116:2216–2233
Kindermann I, Barth C, Mahfoud F, Ukena C, Lenski M, Yilmaz A, Klingel K, Kandolf R, Sechtem U, Cooper LT, Bohm M (2012) Update on myocarditis. J Am Coll Cardiol 59:779–792
Leonea O, Veinot JP, Angelini A, Baandrupd UT, Basso C, Berry G, Bruneval P, Burkeg M, Butanyh J, Calabrese F, d’Amati G, Edwards WD, Fallon JT, Fishbein MC, Gallagher PJ, Halushka MK, McManuso B, Pucci A, Rodriguez R, Saffitz JE, Sheppard MN, Steenbergen C, Stone JR, Tan C, Thiene G, vanderWal AC, Winters GL (2012) 2011 consensus statement on endomyocardial biopsy from the association for European cardiovascular pathology and the society for cardiovascular pathology. Cardiovas Pathol 21:245–274
Krieghoff C, Barten MJ, Hildebrand L, Grothoff M, Lehmkuhl L, Lücke C, Andres C, Nitzsche S (2014) Assessment of sub-clinical acute cellular rejection after heart transplantation: comparison of cardiac magnetic resonance imaging and endomyocardial biopsy. Eur Radiol 24:2360–2371
Yilmaz A, Klingel K, Kandolf R, Sechtem U (2009) Imaging in inflammatory heart disease: from the past to current clinical practice. Hellenic J Cardiol 50:449–460
Baughman KL (2006) Diagnosis of myocarditis: death of Dallas criteria. Circulation 113:593–595
Bock CT, Klingel K, Kandolf R (2010) Human parvovirus B19-associated myocarditis. N Engl J Med 362:1248–1249
Baboonian C, Treasure T (1997) Meta-analysis of the association of enteroviruses with human heart disease. Heart 78:539–543
Cambridge G, MacArthur CGC, Waterson AP, Goodwin JF, Oakley SM (1979) Antibodies to coxsackie B viruses in congestive cardiomyopathy. Brit Heart J 41:692–696
Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang Y-H, Bodurtha J, Gelb B, Ni J, Bricker T, Towbin JA (1994) Acute myocarditis rapid diagnosis by PCR in children. Circulation 90:330–339
Kühl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss H-P (2005) High prevalence of viral genomes and multiple viral infections in the myocardium of adults with “idiopathic” left ventricular dysfunction. Circulation 111:887–893
Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss H-P, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA (2003) Detection of viruses in myocardial tissues by polymerase chain reaction: evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol 42:466–472
Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, Crooks LE (1983) Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol 52:184–188
Ridgway JP (2010) Cardiovascular magnetic resonance physics for clinicians: part I. J Cardiovasc Magn Reson 12:71
Laissy JP, Messin B, Varenne O, Iung B, Karila-Cohen D, Schouman-Claeys E, Steg PG (2002) MRI of acute myocarditis: a comprehensive approach based on various imaging sequences. Chest 122:1638–1648
Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J (2005) Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol 45:1815–1822
Gutberlet M, Spors B, Thomas T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss H-P, Kuhl U (2008) Suspected chronic myocarditis at cardiac mr: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology 246:401–409
Röttgen R, Christiani R, Freyhardt P, Gutberlet M, Schultheiss HP, Hamm B, Kühl U (2011) Magnetic resonance imaging findings in acute myocarditis and correlation with immunohistological parameters. Eur Radiol 21:1259–1266
Voigt A, Elgeti T, Durmus T, Idiz ME, Butler C, Beling M, Schilling R, Klingel K, Kandolf R (2011) Cardiac magnetic resonance imaging in dilated cardiomyopathy in adults—towards identification of myocardial inflammation. Eur Radiol 21:925–935
Lurz P, Eitel I, Adam J, Steiner J, Grothoff M, Desch S, Fuernau G, deWaha S, Sareban M, Luecke C, Klingel K, Kandolf R, Schuler G, Gutberlet M, Thiele H (2012) Diagnostic performance of cmr imaging compared with emb in patients with suspected myocarditis. JACC 5:513–524
Chu GCW, Flewitt JA, Mikami Y, Vermes E, Friedrich MG (2013) Assessment of acute myocarditis by cardiovascular MR: diagnostic performance of shortened protocols. Int J Cardiovasc Imaging 29:1077–1083
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S (2013) T1 mapping for the diagnosis of acute myocarditis using cmr comparison to t2-weighted and late gadolinium enhanced imaging. JACC 6:1048–1058
Šramko M, Kubánek M, Tintěra J, Kautznerová D, Weichet J, Malušková J, Franeková J, Kautzner J (2013) Utility of combination of cardiac magnetic resonance imaging and high-sensitivity cardiac troponin t assay in diagnosis of inflammatory cardiomyopathy. Am J Cardiol 111:258–264
Ferreira VM, Piechnik SK, Dall’Armellina E, Karamitsos TD, Francis JM, Ntusi N, Holloway C, Choudhury RP, Kardos A, Robson MD, Friedrich MG, Neubauer S (2014) Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 16:36
Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K (2014) CMR in patients with severe myocarditis diagnostic value of quantitative tissue markers ncluding extracellular volume imaging. JACC 7:557–575
Luetkens JA, Doerner J, Thomas DK, Dabir D, Gieseke J, Sprinkart AM, Fimmers R (2014) Acute myocarditis: multiparametric cardiac MR imaging. Radiology 273:383–392
Schwab J, Rogg HJ, Pauschinger M, Fessele K, Bareiter T, Bär I, Loose R (2015) Functional and morphological parameters with tissue characterization of cardiovascular magnetic imaging in clinically verified “infarct-like myocarditis”. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 188:363–373
Hinojar R, Foote L, Ucar EA, Jackson T, Jabbour A, Yu CY, McCrohon J, Higgins DM, Carr-White G (2015) Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis a proposed diagnostic algorithm using CMR. JACC 8:37–46
Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL, Block W, Andrie R (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17:154–161
Monney PA, Sekhri N, Burchell T, Knight C, Davies C, Deaner A, Sheaf M, Baithun S, Petersen S, Wragg A, Jain A, Westwood M, Mills P, Mathur A, Mohiddin SA (2011) Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart 97:1312–1318
Mavrogeni S, Spargias C, Bratis C, Kolovou G, Markussis V, Papadopoulou E, Constadoulakis P, Papadimitropoulos M, Douskou M, Pavlides G, Cokkinos D (2011) Myocarditis as a precipitating factor for HF: evaluation and 1-year follow-up using CMR and endomyocardial biopsy. Eur J Heart Fail 13:830–837
Mavrogeni S, Bratis K, Georgakopoulos D, Karanasios E, Kolovou G, Pavlides G, Papadopoulos G (2012) Evaluation of myocarditis in a pediatric population using CMR and endomyocardial biopsy. Int J Cardiol 160:192–195
Stensaeth KH, Hoffmann P, Fossum E, Mangschau A, Sandvik L, Klow NE (2012) Cardiac magnetic resonance visualizes acute and chronic myocardial injuries in myocarditis. Int J Cardiovasc Imaging 2:327–335
Zagrosek A, Abdel-Aty H, Boyé P, Wassmuth R, Messroghli D, Utz W, Rudolph A, Bohl S, Dietz R, Schulz-Menger J (2009) Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC 2:131–138
Francone M, Chimenti C, Galea N, Scopelliti F, Verardo R, Galea R, Carbone I, Catalano C (2014) CMR sensitivity varies with clinical presentation and extent of cell necrosis in biopsy-proven acute myocarditis. JACC 7:254–263
Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R (1998) Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 97:1802–1809
Bohnen S, Radunski UK, Lund GK, Kandolf R, Stehning C, Schnackenburg B, Adam G (2015) Performance of T1 and T2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 8:e003073
Wagner A, Schulz-Menger J, Dietz R, Friedrich MG (2003) Long-term follow-up of patients with acute myocarditis by magnetic resonance imaging. Magma 16:17–20
Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock C-T, Klingel K, Kandolf RR, Sechtem U (2006) Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 114:1581–1590
Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U (2004) Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109:1250–1258
Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U (2008) Coronary vasospasm as the underlying cause for chest pain in patients with PVB19 myocarditis. Heart 94:1456–1463
Yilmaz A, Kindermann I, Kindermann M, Mahfoud F, Ukena C, Athanasiadis A, Hill S, Mahrholdt H, Voehringer M, Schieber M, Klingel K, Kandolf R, Bohm M, Sechtem U (2010) Comparative evaluation of left and right ventricular endomyocardial biopsy differences in complication rate and diagnostic performance. Circulation 122:900–909
Cobelli FD, Pieroni M, Esposito A, Chimenti C, Belloni E, Mellone R, Canu T, Perseghin G, Gaudio C, Maseri A, Frustaci A, Maschio AD (2006) Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J Am Coll Cardiol 47:1649–1654
Yelgec NS, Dymarkowski S, Ganame J, Bogaert J (2007) Value of MRI in patients with a clinical suspicion of acute myocarditis. Eur Radiol 17:2211–2217
Thavendiranathan P, Walls M, Gir S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV (2012) Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 5:102–110
Laissy JP, Hyafil F, Feldman LJ, Juliard J-M, Schouman-Claeys E, Steg PG, Faraggi M (2005) differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology 237:75–82
Banka P, Robinson JD, Uppu SC, Harris MA, Hasbani K, Lai WW, Richmond ME, Fratz S, Jain S (2015) Cardiovascular magnetic resonance techniques and findings in children with myocarditis: a multicenter retrospective study. J Cardiovasc Magn Reson 17:96
Kim RJ, Shah DJ, Judd RM (2003) How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 5:505–514
Baeßler B, Schaarschmidt F, Dick A, Stehning C, Schnackenburg B, Michels G, Maintz D, Bunck AC (2015) Mapping tissue inhomogeneity in acute myocarditis: a novel analytical approach to quantitative myocardial edema imaging by T2-mapping. J Cardiovasc Magn Reson 17:115
Foltz WD, Al-Kwifi O, Sussman MS, Stainsby JA, Wright GA (2003) Optimized spiral imaging for measurement of myocardial T2 relaxation. Magn Reson Med 49:1089–1097
Sparrow P, Amirabadi A, Sussman MS, Paul N, Merchant N (2009) Quantitative assessment of myocardial t2 relaxation times in cardiac amyloidosis. J Cardiovasc Magn Reson 30:942–946
He T, Gatehouse PD, Anderson LJ, Tanner M, Keegan J, Pennell DJ, Firmin DN (2006) Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. J Magn Reson Imaging 24(24):580–585
Gouya H, Vignaux O, LeRoux P, Chanson P, Bertherat J, Bertagna X, Legmann P (2008) Rapidly reversible myocardial edema in patients with acromegaly: assessment with ultrafast T2 mapping in a single-breath-hold MRI sequence. Am J Roentgenol 190:1576–1582
Huang T-Y, Liu Y-J, Stemmer A, Poncelet BP (2007) T2 measurement of the human myocardium using a T2-prepared transient-state TrueFISP sequence. Magn Reson Med 57:960–966
Giri S, Chung Y-C, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP (2009) T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 11:56
Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV (2011) Direct T2 quantification of myocardial edema in acute ischemic injury. JACC 4:269–278
vonKnobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, Niendorf T, Schulz-Menger J (2013) Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 15(53):53
Baeßler B, Schaarschmidt F, Stehning C, Schnackenburg B, Maintza D, Bunck AC (2015) A systematic evaluation of three different cardiac T2-mapping sequences at 1.5 and 3 T in healthy volunteers. Eur J Radiol 84:2161–2170
Blume U, Lockie T, Stehning C, Sinclair S, Uribe S, Razavi R, Schaeffter T (2009) Interleaved T1 and T2 relaxation time mapping for cardiac applications. J Magn Reson Imaging 29:480–487
Sprinkart AM, Luetkens JA, Träber F, Doerner J, Gieseke J, Schnackenburg B, Schmitz G, Thomas D, Homsi R (2015) Gradient spin echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson 17:12
Miller CA, Fildes JE, Ray SG, Doran H, Yonan N, Williams SG, Schmitt M (2013) Non-invasive approaches for the diagnosis of acute cardiac allograft rejection. Heart 99(7):445–453. doi:10.1136/heartjnl-2012-302759
Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R (2013) The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant 32:951–964
Marie PY, Angioi M, Carteaux JP, Escanye JM, Mattei S, Tzvetanov K, Claudon O, Hassan N, Danchin N, Karcher G, Bertrand A, Walker PM, Villemot JP (2001) Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol 37:825–831
Usman AA, Taimen K, Wasielewski M, McDonald J, Shah S, Giri S, Cotts W, McGee E, Gordon R, Collins JD, Mark M, Carr JC (2012) Cardiac magnetic resonance T2 mapping in the monitoring and follow-up of acute cardiac transplant rejection: a pilot study. Circ Cardiovasc Imaging 5:782–790
Miller CA, Naish JH, Shaw SM, Yonan N, Williams SG, Clark D, Bishop PW, Ainslie MP, Borg A, Coutts G, Parke GJM, Ray S, Schmitt M (2014) Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson 16:52
Shanna OP, Maheshwari A, Thaker K (1993) Myocardial Sarcoidosis. Chest 103:253–258
Silverman KJ, Hutchins GM, Bulkley BH (1978) Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 58:1204–1211
Hiraga H, Iwai K, Hiroe M, Omori F, Sekiguchi M, Tachibana T (1993) Guideline for diagnosis of cardiac sarcoidosis: study report on diffuse pulmonary diseases. The Japanese Ministry of Health and Welfare 6:23–24
Patel AR, Klein MR, Chandra S, Spencer KT, DeCara JM, Lang RM, Burke MC, Garrity ER, Hogarth DK (2013) Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail 13:1231–1237
Smedema JP, Snoep G, vanKroonenburgh MPG, vanGeuns RJ, Dassen WRM, Gorgels APM, Crijns HJGM (2005) Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 45:1683–1690
Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB (2009) Detection of myocardial damage in patients with sarcoidosis. Circulation 120:1969–1977
Greulich S, Deluig CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H (2013) CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 6:501–511
Nadel J, Lancefield T, Voskoboinik A, Taylor AJ (2015) Late gadolinium enhancement identified with cardiacmagnetic resonance imaging in sarcoidosis patients is associated with long-term ventricular arrhythmia and sudden cardiac death. Eur Heart J Cardiovasc Imaging 16:634–641
Tadamura E, Yamamuro M, Kubo S, Kanao S, Saga T, Harada M, Ohba M, Hosokawa R, Kimura T, Kita T, Togashi K (2005) Effectiveness of delayed enhanced MRI for identification of cardiac sarcoidosis comparison with radionuclide imaging. Am J Roentgenol 185:110–115
Crouser ED, Ono C, Tran T, He X, Raman SV (2014) Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med 189:109–112
Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S (2008) Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 35:933–941
Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T (2015) Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complete heart block. Heart Rhythm 12:2477–2485
Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor-Avi V (2016) Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging 9:e003738
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Ballocca F, D’Ascenzo F, Moretti C, Omedè P, Cerrato E, Barbero U, Abbate A, Bertero M, Zoccai GB, Gaita F (2015) Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur J. Prev Cardiol 22:1435–1441
Apte M, McGwin G, Vilá LM, Kaslow RA, Alarcón GS, Reveill JD (2008) Associated factors and impact of myocarditis in patients with SLE from LUMINA, a multiethnic US cohort (LV). Rheumatology 47:362–367
Abdel-Aty H, Siegle N, Natusch A, Gromnica-Ihle E, Wassmuth R, Dietz R, Schulz-Menger J (2008) Myocardial tissue characterization in systemic lupus erythematosus: value of a comprehensive cardiovascular magnetic resonance approach. Lupus 17(6):2561–2567
Mavrogeni S, Bratis K, Markussis V, Spargias C, Papadopoulou E, Papamentzelopoulos S, Constadoulakis P, Matsoukas E, Kyrou L, Kolovou G (2013) The diagnostic role of cardiac magnetic resonance imaging in detecting myocardial inflammation in systemic lupus erythematosus. Differentiation from viral myocarditis. Lupus 22:34–43
Singh JA, Woodard PK, Da´vila-Roman VG, Waggoner AD, Gutierrez FR, Zheng J, Eisen SA (2005) Cardiac magnetic resonance imaging abnormalities in systemic lupus erythematosus: a preliminary report. Lupus 14:137–144
Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E (2013) Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging 6:295–301
Zhang Y, Corona-Villalobos CP, Kiani AN, Eng J, Kamel IR, Zimmerman SL, Petri M (2015) Myocardial T2 mapping by cardiovascular magnetic resonance reveals subclinical myocardial inflammation in patients with systemic lupus erythematosus. Int J Cardiovasc Imaging 31:389–397
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, SJ SJJ, Gabriel SE (2005) Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 52:402–411
Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S (2006) Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis 65:1608–1612
Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE (2005) Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum 52:722–732
Giles JT, Fernandes V, Lima JA, Bathon JM (2005) Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther 7:195–207
Kobayashi Y, Giles JT, Hirano M, Yokoe I, Nakajima Y, Bathon JM, Lima JAC, Kobayashi H (2010) Assessment of myocardial abnormalities in rheumatoid arthritis using a comprehensive cardiac magnetic resonance approach: a pilot study. Arthritis Res Ther 12:R171
Mavrogeni S, Bratis K, Sfendouraki E, Papadopoulou E, Kolovou G (2013) Myopericarditis, as the first sign of rheumatoid arthritis relapse, evaluated by cardiac magnetic resonance. Inflamm Allergy Drug Targets 12:206–211
Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Matthews PM, Robson MD, Wordsworth PB, Neubauer S, Karamitsos TD (2015) Diffuse myocardial fibrosis and inflammation in rheumatoid arthritis: insights from CMR T1 mapping. JACC Cardiovasc Imaging 8(5): 526–536
Steen VD, Medsger TA (2000) Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum 43:2437–2444
Champion HC (2008) The heart is scleroderma. Rheum Dis Clin North Am 34:181–190
D’Angelo WA, Fries JF, Masi AT, Shulman LE (1969) Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am J Med 46:428–440
Medsger TJ, Masi A (1973) Survival with Scleroderma. II. A life-table analysis of clinical and demographic factors in 258 male U.S. veteran patients. J Chronic Dis 26:647–660
Tzelepis GE, Kelekis NL, Plastiras SC, Mitseas P, Economopoulos N, Kampolis C, Gialafos EJ, Moyssakis I, Moutsopoulos HM (2007) Pattern and distribution of myocardial fibrosis in systemic sclerosis: a delayed enhanced magnetic resonance imaging study. Arthritis Rheum 56:3827–3836
Hachulla A-L, Launay D, Gaxotte V, Groote Pd, Lamblin N, Devos P, Hatron P-Y, Beregi J-P, Hachulla E (2009) Cardiac magnetic resonance imaging in systemic sclerosis: a cross-sectional observational study of 52 patients. Ann Rheum Dis 68:1878–1884
Rodríguez-Reyna TS, Morelos-Guzman M, Hernández-Reyes P, Montero-Duarte K, Martínez-Reyes C, Reyes-Utrer C, Vazquez J, Morales-Blanhir J (2015) Assessment of myocardial fibrosis and microvascular damage in systemic sclerosis by magnetic resonance imaging and coronary angiotomography. Rheumatology 54:647–654
Sano M, Satoh H, Suwa K, Nobuhara M, Saitoh T, Saotome M, Urushida T, Katoh H, Shimoyama K, Suzuki D, Ogawa N (2015) Characteristics and clinical relevance of late gadolinium enhancement in cardiac magnetic resonance in patients with systemic sclerosis. Heart Vessels 30:779–788
Barison A, Gargani L, Marchi DD, Aquaro GD, Guiducci S, Picano E, Cerinic MM, Pingitore A (2015) Early myocardial and skeletal muscle interstitial remodelling in systemic sclerosis: insights from extracellular volume quantification using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging 16:74–80
Ntusi NAB, Piechnik SK, Francis JM, Ferreira VM, Rai ABS, Matthews PN, Robson MD, Moon J, Wordsworth PB, Neubauer S, Karamitsos TD (2014) Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis: a clinical study using myocardial T1-mapping and extracellular volume quantification. J Cardiovasc Magn Reson 16:21
Kobayashi H, Yokoe I, Hirano M, Nakamura T, Nakajima Y, Fontaine KR, Giles JT, Kobayashi Y (2009) Cardiac magnetic resonance imaging with pharmacological stress perfusion and delayed enhancement in asymptomatic patients with systemic sclerosis. J Rheumatol 36:106–112
DiCesarea E, Battistia S, DiSibioa A, Cipriani P, Giacomelli R, Liakouli V, Ruscitti P, Masciocchia C (2013) Early assessment of sub-clinical cardiac involvement in systemic sclerosis (SSc) using delayed enhancement cardiac magnetic resonance (CE-MRI). Eur J Radiol 82:e268–e273
Schicchi N, Valeri G, Moroncini G, Agliata G, Salvolini L, Gabrielli A, Giovagnoni A (2014) Myocardial perfusion defects in scleroderma detected by contrast-enhanced cardiovascular magnetic resonance. Radiol Med 119:885–894
Mavrogeni SI, Bratis K, Karabela G, Spiliotis G, Wijk K, Hautemann D, Reiber JH, Koutsogeorgopoulou L (2015) Cardiovascular Magnetic Resonance Imaging clarifies cardiac pathophysiology in early, asymptomatic diffuse systemic sclerosis. Inflamm Allergy Drug Targets 14:29–36
Thuny F, Lovric D, Schnell F, Bergerot C, Ernande L, Cottin V, Derumeaux G, Croisille P (2014) Quantification of myocardial extracellular volume fraction with cardiac MR imaging for early detection of left ventricle involvement in systemic sclerosis. Radiology 271:373–380
Acknowledgements
All three authors are employed at the University Hospital of South Manchester. Dr Lagan holds the position of a cardiology research fellow. Dr Schmitt holds the position of a cardiology consultant. Dr Miller holds the position of a cardiology consultant and a clinical lecturer. Dr Miller is supported by a National Institute for Health Research Clinician Scientist Award (CS-2015-15-003).
Funding
No extra funding was received by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All three authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lagan, J., Schmitt, M. & Miller, C.A. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging 34, 35–54 (2018). https://doi.org/10.1007/s10554-017-1063-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1063-9