Abstract
Background
Myocardial T1 and T2 mapping using cardiovascular magnetic resonance (CMR) are promising to improve tissue characterization and early disease detection. This study aimed at analyzing the feasibility of T1 and T2 mapping at 3 T and providing reference values.
Methods
Sixty healthy volunteers (30 males/females, each 20 from 20–39 years, 40–59 years, 60–80 years) underwent left-ventricular T1 and T2 mapping in 3 short-axis slices at 3 T. For T2 mapping, 3 single-shot steady-state free precession (SSFP) images with different T2 preparation times were acquired. For T1 mapping, modified Look-Locker inversion recovery technique with 11 single shot SSFP images was used before and after injection of gadolinium contrast. T1 and T2 relaxation times were quantified for each slice and each myocardial segment.
Results
Mean T2 and T1 (pre-/post-contrast) times were: 44.1 ms/1157.1 ms/427.3 ms (base), 45.1 ms/1158.7 ms/411.2 ms (middle), 46.9 ms/1180.6 ms/399.7 ms (apex). T2 and pre-contrast T1 increased from base to apex, post-contrast T1 decreased. Relevant inter-subject variability was apparent (scatter factor 1.08/1.05/1.11 for T2/pre-contrast T1/post-contrast T1). T2 and post-contrast T1 were influenced by heart rate (p < 0.0001, p = 0.0020), pre-contrast T1 by age (p < 0.0001). Inter- and intra-observer agreement of T2 (r = 0.95; r = 0.95) and T1 (r = 0.91; r = 0.93) were high. T2 maps: 97.7% of all segments were diagnostic and 2.3% were excluded (susceptibility artifact). T1 maps (pre-/post-contrast): 91.6%/93.9% were diagnostic, 8.4%/6.1% were excluded (predominantly susceptibility artifact 7.7%/3.2%).
Conclusions
Myocardial T2 and T1 reference values for the specific CMR setting are provided. The diagnostic impact of the high inter-subject variability of T2 and T1 relaxation times requires further investigation.
Similar content being viewed by others
Background
Cardiovascular magnetic resonance (CMR) provides techniques for non-invasive myocardial tissue characterization. T1 and T2 mapping of the left ventricular myocardium, i.e. quantification of the myocardial T1 and T2 relaxation times, as well as the T1-derived extracellular volume fraction have been demonstrated to add valuable information [1–6]. Most of the experience with myocardial mapping was gained at a magnetic field strength of 1.5 T. Parametric myocardial mapping at 3 T is conceptually appealing due to the signal gain inherent to higher fields, which may be exploited for improved spatial and temporal resolution [7]. Many of the previous studies focused on intra-individual comparison of diseased and remote myocardium. However, T2 and T1 reference values of all myocardial segments may be important to define small focal abnormalities and to identify diffuse tissue changes in the absence of healthy “remote” myocardium. For all these reasons this study scrutinizes myocardial T1 and T2 at 3 T in a large sample of healthy volunteers using state-of-the art mapping techniques.
Methods
Study population
60 healthy volunteers were enrolled into the study (30 men/30 women, equally distributed within 3 age categories (Table 1)). The status “healthy” was based on: i) uneventful medical history, ii) absence of any symptoms indicating cardiovascular dysfunction, iii) normal ECG, iv) normal cardiac dimensions and function proven by cine CMR. v) normal myocardial tissue assessed by late enhancement (LGE). For each volunteer written informed consent was obtained prior to the study, after due approval by the ethical committee of the Charité Medical Faculty (EA2/077/10). All experiments were performed in compliance with the Helsinki Declaration.
CMR examination
All CMR exams were performed with a 3 T system (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) using a 32-channel cardiac RF coil for signal reception, the integrated body RF coil for transmission, and ECG for cardiac gating. Subject-specific, volume-selective first- and second-order B0-shimming based on field maps derived from double-gradient-echo acquisitions was performed to improve static field uniformity. The following CMR protocols were used (Figure 1).
Cine imaging
Steady-state free-precession (SSFP) cine images were obtained during repeated breath-holds in three long axes (horizontal, vertical, and 3-chamber) and in a stack of short axes (SAX) covering the left ventricle (LV) to assess wall motion and for cardiac chamber quantification. Imaging parameters were: repetition time (TR) 3.1 ms, echo time (TE) 1.3 ms, asymmetric echo with factor 0.29, flip angle (FA) 45°, field of view (FOV) (276 × 340)mm2, matrix 156 × 192, slice thickness 6 mm, receiver bandwidth (BW) 704Hz/px, parallel imaging using GRAPPA reconstruction (R = 2), 30 cardiac phases.
T2 mapping
For T2 mapping, data were acquired in basal, mid-ventricular, and apical SAX planes using a T2-prepared single-shot SSFP technique similar to the one described for 1.5 T [2]. For the application on a 3 T platform, the RF pulse length of the SSFP readout module was increased to reduce the SAR deposition and adiabatic T2 preparation pulses were employed to improve the homogeneity of the T2 weighting. Three SSFP images, each with different T2 preparation time (TET2P = 0 ms, 24 ms, 55 ms) were acquired in end-diastole within one breath-hold. Imaging parameters were: TR = 2.4 ms, TE = 1 ms, FA = 70°, FOV = (340 × 278) mm2, matrix = 176 × 144, slice thickness = 6 mm, BW = 1093Hz/px, GRAPPA acceleration factor 2, linear phase encoding scheme. To correct for residual cardiac and respiratory motion between image sets, a non-rigid registration algorithm was used [8]. A pixel-wise myocardial T2-map was generated using unsupervised curve-fitting based on a two-parameter equation [2]. The single shot SSFP readout and use of only three TET2P was chosen to balance accuracy and acquisition time (7 heart cycles) [2, 9].
T1 mapping
For T1 mapping, data were acquired in basal, mid-ventricular, and apical SAX planes before and 10 minutes after administration of 0.2 mmol/kg i.v. gadobutrol (Gadovist®, Bayer Healthcare Germany). Data were obtained in end-diastole using a cardiac-gated, SSFP-based Modified Look-Locker Inversion Recovery (MOLLI) technique [10]. For the application at 3 T, the RF pulse length of the SSFP readout module was increased to reduce the SAR deposition. Imaging parameters were: TR = 2.6-2.7 ms, TE = 1.0-1.1 ms, FA = 35°, FOV = (270 × 360)mm2, matrix = 156 × 208 to 168 × 224, slice thickness = 6 mm, BW = 1045-1028Hz/px, GRAPPA acceleration factor 2, linear phase-encoding ordering, minimum TI of 91 ms. To generate a pixel-wise myocardial T1-map, single-shot SSFP images were acquired at different inversion times (pattern 3-3-5, [10]) and registered [8] prior to a non-linear least-square curve fitting using S(TI) = A - B exp(−TI/T1*) with T1 = T1 × (B/A - 1), where A, B, and T1* are estimated by a three parameter fit [11]. In-plane voxel dimensions were kept isotropic to ensure that partial volume effects are independent of slice rotation.
LGE imaging
LGE imaging was performed in the same planes as SSFP CINE imaging using a segmented inversion-recovery gradient-echo sequence beginning 15 minutes after contrast administration. The inversion time (TI) was repeatedly adjusted to appropriately null the myocardium during the length of LGE image acquisition. Imaging parameters were: TR = 10.5 ms, TE = 5.4 ms, FA = 30°, FOV (350 × 262) mm2, matrix 256 × 162, slice thickness 6 mm, BW 140Hz/px, GRAPPA acceleration factor 2.
CMR image analysis
Image analysis was done using CMR42 (Circle Cardiovascular Imaging, Calgary, Canada).
LV chamber quantification
SSFP cine images were visually evaluated regarding wall motion abnormalities. LV enddiastolic and endsystolic volume and LV mass were determined by manually contouring the endocardial and epicardial borders of the SAX in systole and diastole.
LGE assessment
The absence of LGE was determined qualitatively by visual assessment.
T2 and T1 mapping - qualitative assessment
Each single original image was assessed regarding artifacts caused by susceptibility effects, cardiac or respiratory motion. Each motion-corrected series was evaluated whether the images were correctly aligned. Each map was evaluated whether the original images were transformed to a reasonably appearing map. The presence of artifacts led to the exclusion of all affected myocardial segments. Two experienced readers assessed quality in consensus.
T2 mapping - quantitative assessment
The LV myocardium was delineated by manually contouring the endocardial and epicardial border. We ensured that the region of interest (ROI) was definitely within the myocardium and did not include blood or epicardial fat based. An endocardial and epicardial contour was drawn in one original motion-corrected image. The trabeculated layer and the epicardial border were left out. In doubt, SSFP cine images were consulted. The contours were copied to the other images and adapted to fit in all of these. These final contours were copied to the map. The myocardial ROI was automatically segmented according to the AHA segment model [12]. Results are presented both per segment and averaged per slice.
T1 mapping - quantitative assessment
T1 values were recorded from pre-contrast and post-contrast T1 maps applying the same procedure as for T2.
T2 and T1 mapping - Observer dependency
Intra- and interobserver variability were tested in a subgroup of 20 randomly selected subjects (320 myocardial segments), where one observer measured T2 and pre-contrast T1 values of each LV segment twice with at least 3 months of time between the measurements. A second observer measured T2 and pre-contrast T1 values blinded to the other results.
Statistical analysis
Baseline characteristics are shown as means with standard deviation (SD) or absolute frequencies. Relaxation times are displayed as least-square means with 95% tolerance intervals (90% coverage) and were assessed by slice and by segment using mixed linear models on logarithmic transformed data to ensure normal distributed data. The following co-factors were included into each model to assess their impact on the relaxation times: age (categories), gender, heart rate (binary with split at median), blood pressure and excluded backwards if not significant. For T1 and T2, the scatter factor was provided as back transformed SD, which allows a similar interpretation as the coefficient of variation for non-transformed data. All values presented were back transformed using the exponential to present the data on the original scale. Spearman's correlation coefficients were calculated to evaluate correlations between the co-factors, which may interfere with the modelling. A p-value of less than 0.05 was regarded as statistically significant. Calculations were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA). Intra- and inter-observer dependency was assessed by Bland-Altman analysis and Pearson’s correlation using Prism 5.0 (Graphpad Software, La Jolla, CA, USA).
Results
CMR
All 60 CMR scans were performed without major adverse events. The scans were incomplete in 4 subjects. T2 maps were available for 58 subjects, pre-contrast T1 maps for 59 subjects and post-contrast T1 maps for 57 subjects.
T2 mapping
From 922 segments, 901 (97.7%) were eligible for analysis (Figure 2). A full set of original data and corresponding maps is available as additional file (see Additional file 1). Twenty-one segments (2.3%) were excluded due to a susceptibility artifact (Figures 2 and 3) mainly in the inferior/inferolateral wall (18 out of 21; 85.7%). Exclusion of at least one segment affected 12 out of 58 subjects (20.7%).
T2 relaxation times per slice are shown in Table 2. Mean value was 44.1 ms (base), 45.1 ms (middle) and 46.9 ms (apex). All slices differed significantly (p < 0.0001) with increasing values from base to apex.
T2 values for each myocardial segment are presented in Figure 4. Significant segment-to-segment differences were observed in the basal slice (p = 0.0036) with slightly lower values in the anterior wall compared to inferior. No significant segment-to-segment differences were found for the midventricular (p = 0.5398) and apical slices (p = 0.1367). The distribution of all individual T2 results is illustrated in Figure 5. A relevant inter-subject variability was evident as indicated by a scatter factor of 1.08 (Figure 5 and Table 2).
Mean T 2 and T 1 relaxation times. T2 and T1 relaxation times (ms) for each myocardial segment illustrated as a bulls eye plot that represents the 16 segments of the basal (outer ring), midventricular (middle ring) and apical (central ring) short-axis plane [12]. Results are given as least-square mean and 95% tolerance interval.
Heart rate (ranging from 47 to 102 min-1) was found to significantly influence T2 measurements (p < 0.0001). A heart rate higher than the median (69.5 min-1) was associated with lower T2 values (base: 42.8 ms vs. 45.8 ms; middle: 43.9 ms vs. 46.5 ms; apex: 45.7 ms vs. 48.2 ms). Other tested cofactors including age and gender were not found to be significant.
Pre-contrast T1 mapping
For pre-contrast T1 mapping 938 segments were obtained. 859 (91.6%) were eligible for analysis (Figure 2). A full set of original data and corresponding maps is available as additional file (see Additional file 2). Seventy-two segments (7.7%) were excluded due to a susceptibility artifact and 7 segments (0.7%) due to incorrect motion correction (Figures 2, 6 and 7). In 63 out of 72 segments (87.5%) with susceptibility artifact, the inferior/inferolateral segments were affected. Exclusion of at least one segment affected 34 out of 59 subjects (57.6%).
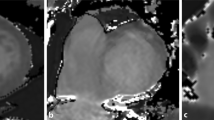
Failed motion correction during T 1 mapping. The original images (upper row) show the regular shape of the LV myocardium (the first five out of eleven images of the complete T1 acquisition are depicted). The motion correction algorithm led to an outbound shift of the anterior and anteroseptal myocardial segment (red arrow in the bottom row). The corresponding map (right image) indicates an inhomogeneous T1 distribution in this area (white arrow).
T1 relaxation times per slice are shown in Table 2. Mean value was 1157.1 ms (base), 1158.7 ms (middle) and 1180.6 ms (apex). Apical T1 relaxation times were significantly larger than basal and midventricular (each p < 0.0001).
T1 values for each myocardial segment are shown in Figure 4. A significant segment-to-segment difference was found for each slice (basal: p < 0.0001; mid: p < 0.0001; apex: p = 0.0153). T1 of the anterior segment was lower than in the other segments. The distribution of all individual T1 results is illustrated in Figure 5. A relevant inter-subject variability was found with a scatter factor of 1.05 (Figure 5, Table 2).
The age categories were found to significantly influence myocardial T1 relaxation times (p < 0.0001). The difference was small between age category 20–39 years and 40–59 years. A clear decrease of T1 relaxation times was observed for subjects ≥ 60 years (Figure 8). Other tested cofactors including heart rate and gender were not found to be significant.
Post-contrast T1 mapping
For post-contrast T1 mapping 841 out of 896 segments (93.9%) were eligible for analysis (Figure 2). Twenty-nine segments (3.2%) were excluded due to a susceptibility artifact, which mainly affected the inferior/inferolateral segments (25 out of 29; 86.2%). Six segments (0.7%) were excluded due to mistriggering (all in one subject). Motion correction failed in one subject in all planes and in one subject in the apical plane leading to an exclusion of 20 segments (2.2%). Exclusion of at least one segment affected 18 out of 56 subjects (32.1%).
T1 relaxation times per slice are shown in Table 2. Mean values of 427.3 ms (base), 411.2 ms (middle) and 399.7 ms (apex) were obtained. All slices differed significantly from each other (base vs. middle: p < 0.0001; base vs. apex p < 0.0001; middle vs. apex: p = 0.0013) with decreasing T1 values from base to apex.
T1 values for each myocardial segment are shown in Figure 4. No significant segment-to-segment differences were observed for each slice (basal: p = 0.4918; mid: p = 0.4741; apex: p = 0.5629). The distribution of all individual T1 results is illustrated in Figure 5. Post-contrast T1-maps revealed a relevant inter-subject variability reflected by a scatter factor of 1.11 (Figure 5, Table 2).
Heart rate was found to significantly influence the post-contrast T1 relaxation time (p = 0.0020) with higher heart rates than the median (69.5 bpm) being associated with lower post-contrast T1 relaxation times (base: 445.7 ms vs. 418.7 ms; middle: 430.1 ms vs. 405.3 ms; apex: 427.6 ms vs. 388.0 ms). Other tested cofactors including age and gender were not found to be significant.
For T2 and pre-contrast T1 mapping inter- and intra-observer analysis demonstrated close agreement (Table 3).
Discussion
This study examined myocardial T1 and T2 mapping techniques at 3 T in a large sample of healthy volunteers. The main findings are: i) T2 and T1 mapping achieve a high grade of diagnostic image quality, although susceptibility artifacts entailed the exclusion of a limited number of myocardial segments from the analysis. ii) Observer dependency of T2 and T1 relaxation time quantification was low. iii) Mean values and 95% tolerance interval of myocardial T2 and T1 relaxation times are presented per slice and per segment and can be used as reference values specific for this MR setting.iii) An inter-subject distribution of T2 and T1 values became apparent and may constitute a limitation to define appropriate cut-offs.
T2 mapping
Previous studies with SSFP-based T2 mapping at 1.5T did not report the exclusion of segments from analysis due to SSFP off-resonance or banding artifacts [2–4]. Hence, this challenge seems to surface at higher field strengths due to the increase in the peak-to-peak B0 inhomogeneity across the heart. The use of an appropriately selected delta frequency may be an option to resolve some artifacts and deserves further systematic investigation. The artifacts mainly affected the inferolateral region, where pathologies like myocarditis may also exhibit their predominant lesion [13]. Despite that, the step from 1.5 T to 3 T for CMR is generally desired due to expected gains in signal, which may be exploited for improved spatial and temporal resolution. This potential promises to enable more detailed insights into cardiac tissue in order to facilitate the early detection of myocardial disease.
T2 relaxation times derived from T2-prepared SSFP imaging in this study are higher compared to a black-blood multi-echo spin-echo approach at 3 T, which provided a mean value of T2 = 39.6m sin the septum [14]. Myocardial T2 reported here was found to be lower versus a mean T2 = 52.2 ms reported for T2 prepared SSFP imaging at 1.5 T [2]. Possible explanations are: i) differences in the pulse sequence design, ii) differences in the spatial resolution, with lower resolution being associated with more partial volume and potentially higher T2 values, and iii) T1 relaxation effects due to higher T1 values at 3 T versus 1.5 T. Generally, myocardial T2 reported in the literature varies substantially, ranging from about 50 ms to 58 ms at 1.5 T [2]. The heterogeneity of data underlines that the measured T2 relaxation time is very sensitive to cofactors and emphasizes the need to generate reference values specific for each technique and imaging setting.
Our results showed that T2 increased from base to apex, which is in accordance with a recent work using a similar mapping technique at 1.5 T [15]. The most probable cause is partial-volume effects that increase towards the apex owing to the curvature of the left ventricle. To encounter this limitation, some groups exclude the apical slice from mapping to omit measurement errors [6]. We tried to minimize this error by carefully drawing the contours in the middle of the myocardium while leaving out the endocardial portion of the myocardium, as well as by using an isotropic spatial resolution as high as possible.
Most of the previous studies reported T2 values averaged over all myocardial segments or only for a midventricular slice. By averaging T2 values over the whole slice or the whole heart, focal T2 deviations may be overlooked. The present study is the largest study, which reports T2 values for each myocardial segment and slice.
As reported for the global T2 values, the segmental T2 values increased from base to apex. In comparison, Markl et al. reported T2 values from 50.5 ms to 51.6 ms in the basal slice and 54.3 ms to 56.1 ms in the apical slice at 1.5 T [15].
The inter-subject variability of absolute T2 values was relatively large both per-slice and per-segment. This finding is in concordance with Thavendiranathan et al., who described T2 values ranging from about 50 ms to 62 ms in healthy controls [4], and with Giri et al., who reported that the apical region showed the most pronounced inter-subject variability [2]. The high inter-subject variability can be considered as the main challenge of T2 mapping, given that the difference in T2 between healthy and injured myocardium has been reported to be relatively small, e.g. 13 ms/11 ms between infarct core/myocarditis and remote myocardium [3, 4].
The association of heart rate and T2 relaxation time is under discussion. Giri et al. reported that the variability between healthy subjects was unrelated to heart rate. Other studies reported lower T2 values in patients with higher heart rate [1, 4]. This may be attributed to the hypothesis that higher heart rates induce pronounced T1 relaxation effects caused by incomplete T1 relaxation, which may affect T2 mapping using a SSFP-based approach. This finding is very relevant for clinical practice as subtle T2 increases may disappear in acutely ill patients with higher heart rates.
T1 mapping
T1 mapping demonstrated diagnostic image quality for the vast majority of myocardial segments. However, a relevant number of myocardial segments had to be excluded due to technical challenges, which would lead to diagnostic uncertainty in a clinical scenario. Previous studies at 1.5 T and 3 T reported lower rates of artifact-related non-diagnostic segments [7, 10, 16, 17]. The explicit source of the artifacts has not been reported in detail in most studies, which renders benchmarking against previous results challenging. A possible contributing factor might be that artifacts are often only visible in the original images - which are used for quality assessment - while they might be not apparent in the final maps. In our study, susceptibility artifacts in the inferolateral region were most frequent.
The pre-contrast T1 values are in concordance with Piechnik et al., who reported T1 = 1169 ms averaged over all myocardial segments [16]. At 3.0 T higher midventricular T1 values (T1 = 1315 ms or T1 = 1286 ms) were reported when using a T1 mapping technique similar to that used in this study [17, 18]. These discrepancies underline that T1 relaxation times are sensitive to many influencing factors.
The myocardial T1 relaxation times reported here can be regarded as reference values specific only for this cohort, time point, mapping technique, type and dosage of contrast media. Further comparisons with other published results are difficult unless an identical study design is used. To provide a context, Lee et al. used 0.15 mmol Gadolinium DTPA and measured a mean T1 of about 550 ms in one midventricular slice after 8.5 min in healthy human subjects at 3 T [17].
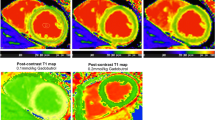
We observed that the pre-contrast T1 times increased from base to apex, whereas the post-contrast T1 values decreased from base to apex. Partial-volume effects owing to the curvature of the left ventricle can most probably explain this finding with blood signal being included into the voxel. While some completely exclude apical T1 maps from analysis [6], we tried to minimize this error by excluding the endocardial portion of the myocardium and by choosing a high isotropic spatial resolution.
In agreement with Kawel et al. we did not observe significant segment-to-segment differences post-contrast [7]. However, pre-contrast T1 values of the anterior segments were lower than T1 observed for the other segments. Interestingly, Piechnik et al. observed the identical pattern with MOLLI at 3 T [16]. Kawel et al. confirmed the presence of regional variability of pre-contrast T1 values inspite of using a different classification into “septal” and “non-septal” myocardium [7]. Although absolute regional difference was small, this finding has to be considered in clinical CMR interpretation as the difference between healthy and abnormal tissue might be in a similar range.
The inter-subject variability of absolute T1 values was notable both per-slice and per-segment, including extreme outliers. This finding is in concordance with other T1 mapping studies reporting pre-contrast T1 values at 1.5 T ranging from 862 ms to 1105 ms in healthy volunteers [19] and a coefficient of variation of 4.5% (pre-contrast) and 7.0% (post-contrast) [18]. The high inter-subject range may be the main challenge of T1 mapping, given that the difference in T1 times between healthy and injured myocardium has been reported to be relatively small depending on the underlying disease. Dall’Armellina et al. reported a mean pre-contrast T1 value of 1257 ± 97 ms for acutely infarcted segments compared to 1196 ± 56 ms for normal unaffected segments at 3 T [20]. In other myocardial diseases like Fabry’s disease or amyloidosis, pre-contrast T1 may already be accurate enough to differentiate cardiac amyloid patients from normals [21].
Post-contrast T1 in the present study was even more variable between subjects than pre-contrast T1, attributable to the many factors with influence on the contrast kinetics (e.g. patient weight, hematocrit, renal function). Miller et al. recently demonstrated that even though isolated post-contrast T1 measurement showed significant within-subject correlation with histological collagen volume fraction, the between-subject correlations were not significant. Hence, isolated post-contrast T1 measurement seems to be insufficient for assessing extracellular volume fraction [22].
Aging was found to be associated with decreasing pre-contrast T1 values. This is an interesting aspect that may reflect early age-dependent alterations of myocardial texture. Dall’Armellina et al. and Ugander et al. showed that pre-contrast T1 times were increased in acute myocardial ischemia [20, 23]. Dass et al. reported increase in pre-contrast T1 in cardiomyopathies. Hence, the present reduction of pre-contrast T1 with age may sound contradictory [24]. In contrast, in a rat model, diffuse myocardial fibrosis was associated with a non-significant trend towards lower pre-contrast T1 values [25]. Therefore our data are stimulating to further analyze the value of pre-contrast T1 mapping in non-ischemic heart disease in future.
Conclusion
In conclusion, myocardial T2 and T1 mapping at 3 T are feasible with a good diagnostic image quality, although susceptibility artifacts related to the magnetic field strength of 3 T triggered exclusion of myocardial segments from analysis. This study provides reference values for myocardial T2 and T1 relaxation times per slice and per segment for the specific MR setting, which were deduced from a large cohort of healthy volunteers. With this approach a relatively high inter-subject distribution became apparent, which may constitute a relevant challenge for the definition of cut-offs that differentiate healthy from diseased myocardium in clinical practice.
Study limitations
i) As hematocrit was not measured in this study, its effect on T2 and T1 relaxation times could not be assessed. ii) Whereas observer variability to assess T2 and T1 relaxation times was low, the inter-scan variability was not assessed and deserves further investigation. iii) Regarding T1 estimation by the applied MOLLI technique, there are known limitations to inversion efficiency [26] and to evaluation of magnitude based data. The inversion efficiency is also dependent on T2. At the time the study was designed, an improved inversion pulse designed for myocardial T1 mapping tailored for myocardial T2 was not available yet. The limitations of evaluating T1 based on magnitude images are described in a recent publication [27]. By the time the study was designed, the proposed phase-sensitive recon was not yet available on our system.
References
Messroghli DR, Niendorf T, Schulz-Menger J, Dietz R, Friedrich MG: T1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003, 5: 353-9. 10.1081/JCMR-120019418.
Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP: T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009, 11: 56-10.1186/1532-429X-11-56.
Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, Raman SV: Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011, 4: 269-78. 10.1016/j.jcmg.2010.09.023.
Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S, Simonetti OP, Raman SV: Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. 2012, 5: 102-10. 10.1161/CIRCIMAGING.111.967836.
Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC: Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010, 122: 138-44. 10.1161/CIRCULATIONAHA.109.930636.
Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB: Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012, 126: 1206-16. 10.1161/CIRCULATIONAHA.111.089409.
Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA: T1 mapping of the myocardium: Intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012, 14: 27-10.1186/1532-429X-14-27.
Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P: Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012, 67: 1644-55. 10.1002/mrm.23153.
MacFall JR, Riederer SJ, Wang HZ: An analysis of noise propagation in computed T2, pseudodensity, and synthetic spin-echo images. MedPhys. 1986, 13: 285-92.
Messroghli DR, Greiser A, Frohlich M, Dietz R, Schulz-Menger J: Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007, 26: 1081-6. 10.1002/jmri.21119.
Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP: Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004, 52: 141-6. 10.1002/mrm.20110.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS: Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002, 105: 539-42. 10.1161/hc0402.102975.
Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J: Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005, 45: 1815-22. 10.1016/j.jacc.2004.11.069.
Guo H, Au WY, Cheung JS, Kim D, Jensen JH, Khong PL, Chan Q, Chan KC, Tosti C, Tang H, Brown TR, Lam WW, Ha SY, Brittenham GM, Wu EX: Myocardial T2 quantitation in patients with iron overload at 3 Tesla. J Magn Reson Imaging. 2009, 30: 394-400. 10.1002/jmri.21851.
Markl M, Rustogi R, Galizia M, Goyal A, Collins J, Usman A, Jung B, Foell D, Carr J: Myocardial T2-mapping and velocity mapping: Changes in regional left ventricular structure and function after heart transplantation. Magn Res Med. 2012, epub ahead of print
Piechnik SK, Ferreira VM, Dall'Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD: Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010, 12: 69-10.1186/1532-429X-12-69.
Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA: Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011, 13: 75-10.1186/1532-429X-13-75.
Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA: T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012, 14: 26-10.1186/1532-429X-14-26.
Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU: Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution–reproducibility study. Radiology. 2006, 238: 1004-12. 10.1148/radiol.2382041903.
Dall'Armellina E, Piechnik SK, Ferreira VM, Si QL, Robson MD, Francis JM, Cuculi F, Kharbanda RK, Banning AP, Choudhury RP, Karamitsos TD, Neubauer S: Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012, 14: 15-10.1186/1532-429X-14-15.
Karamitsos TD, Piechnik SK, Banypersad SM, Fontana M, Ntusi NB, Ferreira VM, Whelan CJ, Myerson SG, Robson MD, Hawkins PN, Neubauer S, Moon JC: Noncontrast t1 mapping for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. 2013, 6: 488-97. 10.1016/j.jcmg.2012.11.013.
Miller CA, Naish J, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, Moon JC, Greiser A, Parker GJ, Schmitt M: Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013, 6: 373-83. 10.1161/CIRCIMAGING.112.000192.
Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, Shah S, Greiser A, Kellman P, Arai AE: Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012, 5: 596-603. 10.1016/j.jcmg.2012.01.016.
Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S: Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012, 5: 726-33. 10.1161/CIRCIMAGING.112.976738.
Messroghli DR, Nordmeyer S, Dietrich T, Dirsch O, Kaschina E, Savvatis K, Oh-I D, Klein C, Berger F, Kuehne T: Assessment of diffuse myocardial fibrosis in rats using small-animal Look-Locker inversion recovery T1 mapping. Circ Cardiovasc Imaging. 2011, 4: 636-40. 10.1161/CIRCIMAGING.111.966796.
Kingsley PB, Ogg RJ, Reddick WE, Steen RG: Correction of errors caused by imperfect inversion pulses in MR imaging measurement of T1 relaxation times. Magn Reson Imaging. 1998, 16: 1049-55. 10.1016/S0730-725X(98)00112-X.
Xue H, Greiser A, Zuehlsdorff S, Jolly MP, Guehring J, Arai AE, Kellman P: Phase-sensitive inversion recovery for myocardial T1 mapping with motion correction and parametric fitting. Magn Reson Med. 2013, 69: 1408-20. 10.1002/mrm.24385.
Acknowledgements
The authors wish to acknowledge the technicians Kerstin Kretschel, Evelyn Polzin, Denise Kleindienst and Franziska Neumann for assisting in acquiring the CMR data, and the study nurses Elke Nickel-Szczech and Antje Els for assisting in the organization of the CMR scans.
This project was supported by a grant of the Else Kröner-Fresenius Stiftung (Bad Homburg, Germany).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The co-author A. Greiser is employee of Siemens Healthcare, Germany. The other authors declare that they have no competing interests.
Authors’ contributions
FvKB defined the design of the study, headed its coordination, acquired the image data, read the images, assisted in statistical analysis and drafted the manuscript. MP contributed to the analysis and interpretation of the data and was involved in drafting the manuscript. MD made contributions to acquisition of data and was involved in drafting the manuscript. RW and AG made substantial contributions to the analysis and interpretation of the data and were involved in drafting the manuscript. CS participated in the design of the study, performed the statistical analysis and was involved in drafting the manuscript. TN made substantial contributions to the acquisition, analysis and interpretation of the data and was involved in drafting the manuscript. JSM defined the design of the study, headed its coordination, assisted in statistical analysis and interpretation of the data, and drafted the manuscript. All authors read and approved the final manuscript.
Electronic supplementary material
12968_2013_3306_MOESM1_ESM.tiff
Additional file 1: T 2 -mapping. A full set of T2-weighted SSFP single shot images with 3 different T2 preparation times and the corresponding T2 maps from the basal, midventricular and apical slice. (TIFF 4 MB)
12968_2013_3306_MOESM2_ESM.tiff
Additional file 2: T 1 -mapping. A full set of T1-weighted SSFP single-shot images and the corresponding pre-contrast T1 maps from the basal, midventricular and apical slice. (TIFF 6 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
von Knobelsdorff-Brenkenhoff, F., Prothmann, M., Dieringer, M.A. et al. Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 15, 53 (2013). https://doi.org/10.1186/1532-429X-15-53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1532-429X-15-53