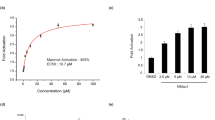
Abstract
Purpose
More than 90% of the breast cancer deaths occur due to the metastasis of the cancer cells to secondary organ sites. Increased Glucose-regulated protein 78 (GRP78) expression is critical for epithelial–mesenchymal transition (EMT) and invasion in breast cancer resulting in poor patient survival outcomes. Therefore, there is an urgent need of potential inhibitors of GRP78 for the abrogation of invasion and metastasis in breast cancer.
Methods
We investigated the effect of IKM5 (2-(1-(1H-indol-3-yl)octyl)-3-hydroxy-6-(hydroxymethyl)-4H-pyran-4-one) (a novel Indolylkojyl methane analogue) on invasion abilities of human breast cancer cells employing invadopodia formation, Matrigel invasion assays, and mouse models for metastasis. The mechanism underlying the anti-invasive effect of IKM5 was examined through molecular docking, immunoblotting, immunocytochemistry, co-immunoprecipitation analysis, siRNA silencing, and sub-cellular fractionation studies.
Results
Treatment with IKM5 at its sub-toxic concentration (200 nM) suppressed invasion and invadopodia formation, and growth factor-induced cell scattering of aggressive human breast cancer MDA-MB-231, MDA-MB-468, and MCF7 cells. IKM5 spontaneously binds to GRP78 (Ki = 1.35 µM) and downregulates its expression along with the EMT markers MMP-2, Twist1, and Vimentin. Furthermore, IKM5 amplified the expression and nuclear translocation of tumor suppressor Par-4 to control NF-kB-mediated pro-EMT activities. Interestingly, IKM5 disrupts the interaction between GRP78 and TIMP-1 by inhibiting GRP78 in a Par-4-dependent manner. Moreover, IKM5 inhibited tumor growth and lung metastasis at a safe dose of 30 mg/kg/body weight.
Conclusion
Our study warrants IKM5, a potential anticancer agent that can abrogate invasion and metastasis, suggesting its clinical development for the treatment of patients with advanced breast cancer.






Similar content being viewed by others
Abbreviations
- EMT:
-
Epithelial-mesenchymal transition
- GRP78:
-
Glucose-regulated protein 78
- MMP-2:
-
Matrix metalloproteinase 2
- TIMP-1:
-
Tissue inhibitor of metalloproteases 1
- Par-4:
-
Prostate apoptosis response 4
- NF-kB:
-
Nuclear factor kappa B
- ER:
-
Endoplasmic reticulum
- KA:
-
Kojic acid
References
Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J, Cristofanilli M, Dontu G, Bidaut L, Valero V (2009) Breast cancer metastasis: challenges and opportunities. Cancer Res 69(12):4951–4953
Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695
Jin X, Mu P (2015) Targeting breast cancer metastasis. Breast Cancer 9:S25460
Lee AS (2007) GRP78 induction in cancer: therapeutic and prognostic implications. Can Res 67(8):3496–3499
Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, Jhaveri N, Zhu G, Ye R, Liu Z (2011) A critical role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Can Res 71(8):2848–2857
Zhao G, Kang J, Jiao K, Xu G, Yang L, Tang S, Zhang H, Wang Y, Nie Y, Wu K (2015) High expression of GRP78 promotes invasion and metastases in patients with esophageal squamous cell carcinoma. Dig Dis Sci 60(9):2690–2699
Yuan XP, Dong M, Li X, Zhou JP (2015) GRP78 promotes the invasion of pancreatic cancer cells by FAK and JNK. Mol Cell Biochem 398(1–2):55–62
Lamouille S, Xu J, Derynck R (2012) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Investig 119(6):1420–1428
Bentley R (2006) From miso, sake and shoyu to cosmetics: a century of science for kojic acid. Nat Prod Rep 23(6):1046–1062
Chen Y-H, Lu P-J, Hulme C, Shaw AY (2013) Synthesis of kojic acid-derived copper-chelating apoptosis inducing agents. Med Chem Res 22(2):995–1003
Yoo DS, Lee J, Choi SS, Rho HS, Cho DH, Shin WC, Cho JY (2010) A modulatory effect of novel kojic acid derivatives on cancer cell proliferation and macrophage activation. Die Pharmazie-An Int J Pharm Sci 65(4):261–266
Nawarak J, Huang-Liu R, Kao S-H, Liao H-H, Sinchaikul S, Chen S-T, Cheng S-L (2008) Proteomics analysis of kojic acid treated A375 human malignant melanoma cells. J Proteome Res 7(9):3737–3746
Sharma DK, Rah B, Lambu MR, Hussain A, Yousuf SK, Tripathi AK, Singh B, Jamwal G, Ahmed Z, Chanauria N (2012) Design and synthesis of novel N, N’-glycoside derivatives of 3, 3’-diindolylmethanes as potential antiproliferative agents. MedChemComm 3(9):1082–1091
Nayak D, Amin H, Rah B, Rasool R, Sharma D, Gupta AP, Kushwaha M, Mukherjee D, Goswami A (2015) A therapeutically relevant, 3, 3’-diindolylmethane derivative NGD16 attenuates angiogenesis by targeting glucose regulated protein, 78 kDa (GRP78). Chem Biol Interact 232:58–67
Wisniewska M, Karlberg T, Lehtio L, Johansson I, Kotenyova T, Moche M, Schuler H (2010) Crystal structures of the ATPase domains of four human Hsp70 isoforms: HSPA1L/Hsp70-hom, HSPA2/Hsp70-2, HSPA6/Hsp70B’, and HSPA5/BiP/GRP78. PLoS ONE 5(1):e8625
Viewer S-PDB (2001) Kaplan W; Littlejohn TG. Brief Bioinform 2(2):195–197
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791
Laskowski RA, Swindells MB (2011) LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model 51(10):2778–2786
DeLano WL (2002) The PyMOL molecular graphics system. http://pymol.org
Amin H, Nayak D, Chakraborty S, Kumar A, Yousuf K, Sharma PR, Ahmed Z, Sharma N, Magotra A, Mukherjee D (2016) Par-4 dependent modulation of cellular β-catenin by medicinal plant natural product derivative 3-azido Withaferin A. Mol Carcinog 55(5):864–881
Nayak D, Kumar A, Chakraborty S, ur Rasool R, Amin H, Katoch A, Gopinath V, Mahajan V, Zilla MK, Rah B (2017) Inhibition of Twist1-mediated invasion by Chk2 promotes premature senescence in p53-defective cancer cells. Cell Death Differ 24(7):1275
Rah B, Rasool Ru, Nayak D, Yousuf SK, Mukherjee D, Kumar LD, Goswami A (2015) PAWR-mediated suppression of BCL2 promotes switching of 3-azido withaferin A (3-AWA)-induced autophagy to apoptosis in prostate cancer cells. Autophagy 11(2):314–331
Rasool RU, Nayak D, Chakraborty S, Faheem MM, Rah B, Mahajan P, Gopinath V, Katoch A, Iqra Z, Yousuf SK (2017) AKT is indispensable for coordinating Par-4/JNK cross talk in p21 downmodulation during ER stress. Oncogenesis 6(5):e341
Ostacolo L, Marra M, Ungaro F, Zappavigna S, Maglio G, Quaglia F, Abbruzzese A, Caraglia M (2010) In vitro anticancer activity of docetaxel-loaded micelles based on poly (ethylene oxide)-poly (epsilon-caprolactone) block copolymers: do nanocarrier properties have a role? J Control Release 148(2):255–263
Anderson VE, Walton MI, Eve PD, Boxall KJ, Antoni L, Caldwell JJ, Aherne W, Pearl LH, Oliver AW, Collins I (2011) CCT241533 is a potent and selective inhibitor of CHK2 that potentiates the cytotoxicity of PARP inhibitors. Cancer Res 71(2):463–472
Goswami A, Burikhanov R, de Thonel A, Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T, Rangnekar VM (2005) Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol Cell 20(1):33–44
El-Guendy N, Zhao Y, Gurumurthy S, Burikhanov R, Rangnekar VM (2003) Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol Cell Biol 23(16):5516–5525
Burikhanov R, Zhao Y, Goswami A, Qiu S, Schwarze SR, Rangnekar VM (2009) The tumor suppressor Par-4 activates an extrinsic pathway for apoptosis. Cell 138(2):377–388
Maloisel F, Kurtz JE, Andres E, Gorodetsky C, Dufour P, Oberling F (1995) Platin salts-induced hemolytic anemia: cisplatin-and the first case of carboplatin-induced hemolysis. Anticancer Drugs 6(2):324–326
Woodward JKL, Neville-Webbe HL, Coleman RE, Holen I (2005) Combined effects of zoledronic acid and doxorubicin on breast cancer cell invasion in vitro. Anticancer Drugs 16(8):845–854
Chaudhry P, Singh M, Parent S, Asselin E (2012) Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation. Mol Cell Biol 32(4):826–839
Sharma SP (2006) GRP78 as potential predictor of chemoresistance. Lancet Oncol 7(10):800
Lee E, Nichols P, Spicer D, Groshen S, Mimi CY, Lee AS (2006) GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Can Res 66(16):7849–7853
Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS (2003) Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors role of ATP binding site in suppression of caspase-7 activation. J Biol Chem 278(23):20915–20924
Avril T, Vauleon E, Chevet E (2017) Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 6(8):e373
Kern J, Untergasser G, Zenzmaier C, Sarg B, Gastl G, Gunsilius E, Steurer M (2009) GRP-78 secreted by tumor cells blocks the antiangiogenic activity of bortezomib. Blood 114(18):3960–3967
Pyrko P, Schönthal AH, Hofman FM, Chen TC, Lee AS (2007) The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Can Res 67(20):9809–9816
Uckun FM, Qazi S, Ozer Z, Garner AL, Pitt J, Ma H, Janda KD (2011) Inducing apoptosis in chemotherapy-resistant B-lineage acute lymphoblastic leukaemia cells by targeting HSPA5, a master regulator of the anti-apoptotic unfolded protein response signalling network. Br J Haematol 153(6):741–752
Daneshmand S, Quek ML, Lin E, Lee C, Cote RJ, Hawes D, Cai J, Groshen S, Lieskovsky G, Skinner DG (2007) Glucose-regulated protein GRP78 is up-regulated in prostate cancer and correlates with recurrence and survival. Hum Pathol 38(10):1547–1552
Scriven P, Coulson S, Haines R, Balasubramanian S, Cross S, Wyld L (2009) Activation and clinical significance of the unfolded protein response in breast cancer. Br J Cancer 101(10):1692
Roller C, Maddalo D (2013) The molecular chaperone GRP78/BiP in the development of chemoresistance: mechanism and possible treatment. Front Pharmacol 4:10
Sun X, Huo X, Luo T, Li M, Yin Y, Jiang Y (2011) The anticancer flavonoid chrysin induces the unfolded protein response in hepatoma cells. J Cell Mol Med 15(11):2389–2398
Umeda Y, Chijiwa S, Furihata K, Furihata K, Sakuda S, Nagasawa H, Watanabe H, Shin-ya K (2005) Prunustatin A, a novel GRP 78 molecular chaperone down-regulator isolated from Streptomyces violaceoniger. J Antibiot 58(3):206–209
Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S (2006) 3, 3’-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis 27(4):717–728
Sun S, Han J, Ralph WM Jr, Chandrasekaran A, Liu K, Auborn KJ, Carter TH (2004) Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell Stress Chaperones 9(1):76–87
Tsai Y-L, Zhang Y, Tseng C-C, Stanciauskas R, Pinaud F, Lee AS (2015) Characterization and mechanism of stress-induced translocation of 78-kilodalton glucose-regulated protein (GRP78) to the cell surface. J Biol Chem 290(13):8049–8064
Kang BR, Yang S-H, Chung B-R, Kim W, Kim Y (2016) Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci Rep 6:34922
Shani G, Fischer WH, Justice NJ, Kelber JA, Vale W, Gray PC (2008) GRP78 and Cripto form a complex at the cell surface and collaborate to inhibit transforming growth factor Î2 signaling and enhance cell growth. Mol Cell Biol 28(2):666–677
Zhang S, Li L, Lin J-Y, Lin H (2003) Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol 9(5):899–904
Lu H, Cao X, Zhang H, Sun G, Fan G, Chen L, Wang S (2014) Imbalance between MMP-2, 9 and TIMP-1 promote the invasion and metastasis of renal cell carcinoma via SKP2 signaling pathways. Tumor Biol 35(10):9807–9813
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141(1):52–67
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z (2000) Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2(10):737–744
Katoch A, Suklabaidya S, Chakraborty S, Nayak D, Rasool RU, Sharma D, Mukherjee D, Faheem MM, Kumar A, Sharma PR (2018) Dual Role of Par-4 in abrogation of EMT and switching on Mesenchymal to Epithelial Transition (MET) in metastatic pancreatic cancer cells. Mol Carcinog 57(9):1102–1115
Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29(3):405–434
Li C-W, Xia W, Huo L, Lim S-O, Wu Y, Hsu JL, Chao C-H, Yamaguchi H, Yang N-K, Ding Q (2012) Epithelial-mesenchymal transition induced by TNF-α requires NF-kB-mediated transcriptional upregulation of Twist1. Can Res 72(5):1290–1300
Acknowledgement
We thank our Director, Dr R. A. Vishwakarma (IIIM, Jammu, India), for his encouragement and support to accomplish this work. The study was funded by institutional internal grant (MLP-6002) from Council of Scientific & Industrial Research (CSIR), Govt. of India with publication number IIIM/2297/2019. The authors acknowledge CSIR and Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India for providing fellowships to the research scholars.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies conducted on human participants by any of the co-authors. Animal studies were approved by the Institutional Animal Ethics Committee (IAEC), CPCSEA, Indian Institute of Integrative Medicine, Jammu, India and performed following all the necessary guidelines of IAEC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nayak, D., Katoch, A., Sharma, D. et al. Indolylkojyl methane analogue IKM5 potentially inhibits invasion of breast cancer cells via attenuation of GRP78. Breast Cancer Res Treat 177, 307–323 (2019). https://doi.org/10.1007/s10549-019-05301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05301-0