Abstract
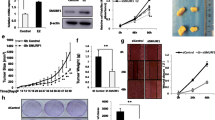
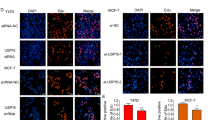
ERα function is crucial for the development of normal mammary gland as well as in the process of progression of breast cancer cells. Signals that target receptor levels contribute to regulate estrogens effects in the cells. An intricate cross-regulation has been documented between ERα and TGF-β down-stream molecules: SMAD2, SMAD3, and SMAD4, that can bind ERα and regulate their signaling. Thus, identification of natural anticancer drugs able to influence the latter molecule might provide alternative choices for breast cancer treatment. Taking into account our previous published data we wanted to study the effect of 5-Methoxypsoralen (bergapten) on ERα and on TGF-β pathway. We reported that bergapten, a coumarin containing compound, effectively depletes ERα in MCF-7 breast cancer sensitive cells and in tamoxifen-resistant clone. The decrease of ERα protein after bergapten treatment results from the ubiquitine–proteasome pathway as demonstrated by the use of MG-132. IP experiments with ER antibody, demonstrated that the protein has physical interaction with SMAD4 and poly-ubiquitine and the amount of ubiquitinated receptor, linked to SMAD4, is greater under bergapten. The crucial role played by SMAD4, in this process, emerges from the observation that in breast cancer cells, silencing of SMAD4, resulted in increased expression of endogenous ERα in both control and bergapten-treated cells, compared to wild- type cells. The same results were confirmed in siRNA TGF-β RII cells. The results suggest a novel negative regulation of ERα by TGF-β/SMAD4 in breast cancer cells and indicate that the SMAD4 protein is involved in the degradation of ERα induced by bergapten. We propose that bergapten may efficiently act as a natural antitumoral agent, able to deplete ERα from breast cancer tamoxifen-sensitive and resistant cells, thereby retraining the effect of membrane signals targeting ERα and in such way its mitogenic potentiality.










Similar content being viewed by others
Abbreviations
- B:
-
Bergapten
- 5-MOP:
-
5-Methoxypsoralen
- ERα:
-
Estrogen receptor α
- TGF-β:
-
Trasforming growth factor beta
- MAPK:
-
Mitogen-activated protein kinase
- Poly Ub:
-
Poly-ubiquitin
References
Furr BJ, Jordan VC (1984) The pharmacology and clinical uses of tamoxifen. Pharmacol Ther 2:127–205
Osborne CK, Coronado-Heinsohn EB, Hilsenbeck SG, Mc-Cue BL, Wakeling AE, McClelland RA, Manning DL, Nicholson RI (1995) Comparison of the effects of a pure steroidal antiestrogen with those of tamoxifen in a model of human breast cancer. J Natl Cancer Inst 87:746–750
Johnston SR, Head J, Pancholi S, Detre S, Martin LA, Smith IE, Dowsett M (2003) Integration of signal transduction inhibitors with endocrine therapy: an approach to overcoming hormone resistance in breast cancer. Clin Cancer Res 9(1 Pt 2):524S–532S
Morrow M, Jordan VC (1993) Molecular mechanisms of resistance to tamoxifen therapy in breast cancer. Arch Surg 128:1187–1191
Zhang Y, Moerkens M, Ramaiahgari S, de Bont H, Price L, Meerman J, van de Water B (2011) Elevated insulin-like growth factor 1 receptor signaling induces antiestrogen resistance through the MAPK/ERK and PI3 K/Akt signaling routes. Breast Cancer Res 3:R52
Ma T, Liu L, Xue H, Li L, Han C, Wang L, Chen Z, Liu G (2008) Chemical library and structure-activity relationships of 11-demethyl-12-oxo calanolide a analogues as anti-HIV-1 agents. J Med Chem 5:1432–1446
Kidane AG, Salacinski H, Tiwari A, Bruckdorfer KR, Seifalian AM (2004) Anticoagulant and antiplatelet agents. Their clinical and device application (s) together with usages to engineer surface. Biomacromolecules 5:798–813
Appendino G, Percalli E, Fuzzati N, Arnoldi L, Stavri M, Gibbons S, Ballero M, Maxia AJ (2004) Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis). J Nat Prod 12:2108–2110
Kontogiorgis CA, Hadjipavlou LD (2004) Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg Med Chem Lett 3:611–614
Sashidhara KV, Rosaiah JN, Kumar A, Bhatia G, Khanna AK (2010) Synthesis of novel benzocoumarin derivatives as lipid lowering agents. Bioorg Med Chem Lett 10:3065–3069
Kostova I (2005) Synthetic and natural coumarins as cytotoxic agents. Curr Med Chem Anticancer Agents 1:29–46
Musa MA, Cooperwood JS, Khan MO (2008) A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr Med Chem 26:2664–2679
Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S (2010) Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett 24:7205–7211
Panno ML, Giordano F, Palma MG, Bartella V, Rago V, Maggiolini M, Sisci D, Lanzino M, De Amicis F, Andò S (2009) Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr Cancer Drug Targets 4:469–481
Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK (1988) Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res 48:3898–3904
Matsuda T, Yamamoto T, Muraguchi A, Saatcioglu F (2001) Cross-talk between transforming growth factor-beta and estrogen receptor signaling through Smad3. J Biol Chem 276:42908–42914
Ito I, Hanyu A, Wayama M, Goto N, Katsuno Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, Hirota R, Murayama A, Kimura K, Imamura T, Yanagisawa J (2010) Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J Biol Chem 19:14747–14755
D Amicis F, Giordano F, Vivacqua A, Pellegrino M, Panno ML, Tramontano D, Fuqua SA, Andò (2011) Resveratrol through NF-Y/p53/Sin3/HDAC1 complex phosphorylation, inhibits estrogen receptor alpha gene expression via p38MAPK/CK2 signaling in human breast cancer cells. FASEB J 25(10):3695–3707
Bartella V, Rizza P, Barone I, Zito D, Giordano F, Giordano C, Catalano S, Mauro L, Sisci D, Panno ML, Fuqua SAW, Andò S (2012) Estrogen receptor beta binds Sp1 and recruits a corepressor complex to the estrogen receptor alpha gene promoter. Breast Cancer Res Treat 134(2):569–581
Guido C, Panza S, Santoro M, Avena P, Panno ML, Perrotta I, Giordano F, Casaburi I, Catalano S, De Amicis F, Sotgia F, Lisanti MP, Andò S, Aquila S (2012) Estrogen receptor beta (ERβ) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle 11:2911–2921
Sisci D, Middea E, Morelli C, Lanzino M, Aquila S, Rizza P, Catalano S, Casaburi I, Maggiolini M, Andò S (2010) 17 beta-Estradiol enhances alpha(5) integrin subunit gene expression through ER alpha-Sp1 interaction and reduces cell motility and invasion of ER alpha-positivbreast cancer cells. Breast Cancer Res Treat 124:63–770
Panno ML, Giordano F, Mastroianni F, Morelli C, Brunelli E, Palma MG, Pellegrino M, Aquila S, Miglietta A, Mauro L, Bonofiglio D, Andò S (2006) Evidence that low doses of Taxol enhance the functional transactivatory properties of p53 on p21 waf promoter in MCF-7 breast cancer cells. FEBS Lett 580:2371–2380
Catalano S, Giordano C, Rizza P, Gu G, Barone I, Bonofiglio D, Giordano f, malivindi R, Faccione D, lanzino M, De Amicis F, Andò S (2009) Evidence that leptin through STAT and CREB signalling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J Cell Physiol 218(3):490–500
Giordano C, Cui Y, Barone I, Andò S, Mancini MA, Berno V, Fuqua SWA (2010) Growth factor-induced resistance to tamoxifen is associated with a mutation of estrogen receptor α and its phosphorylation at serine 305. Breast Cancer Res Treat 119:71–85
Chen S, Cho M, Karlsberq K, Zhou D, Yuan YC (2004) Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J Biol Chem 46:48071–48078
Macaulay VM, Bates PJ, McLean MJ, Rowlands MG, Jenkins TC, Ashworth A, Neidle S (1995) Inhibition of aromatase expression by a psoralen-linked triplex-forming oligonucleotide targeted to a coding sequence. FEBS Lett 372:222–228
Wu L, Wu Y, Gathings B, Wan M, Li X, Grizzle W, Liu Z, Lu C, Mao Z, Cao X (2003) Smad4 as a transcription corepressor for estrogen receptor alpha. J Biol Chem 17:15192–15200
Ren Y, Wu L, Frost AR, Grizzle W, Cao X, Wan M (2009) Dual effects of TGF-beta on ER alpha-mediated estrogenic transcriptional activity in breast cancer. Mol cancer 8:1–11
Petrel TA, Brueggemeier RW (2003) Increased proteasome-dependent degradation of estrogen receptor-alpha by TGF-beta1 in breast cancer cell lines. J Cell Biochem 88:181–190
Acknowledgments
This study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC 2011, grant IG 11595) and PRIN grant 2008; we thank Dr. P. Cicirelli for technical assistance, University of Calabria, Cosenza.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. L. Panno & F. Giordano contributed equally to this work.
Rights and permissions
About this article
Cite this article
Panno, M.L., Giordano, F., Rizza, P. et al. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res Treat 136, 443–455 (2012). https://doi.org/10.1007/s10549-012-2282-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2282-3