Abstract
The Native American Pima population has the highest incidence of insulin resistance (IR) and type 2 diabetes mellitus (T2DM) of any reported population, but the pathophysiologic mechanism is unknown. Genetic studies in Pima Indians have linked acyl-CoA dehydrogenase 10 (ACAD10) gene polymorphisms, among others, to this predisposition. The gene codes for a protein with a C-terminus region that is structurally similar to members of a family of flavoenzymes—the acyl-CoA dehydrogenases (ACADs)—that catalyze α,β-dehydrogenation reactions, including the first step in mitochondrial FAO (FAO), and intermediary reactions in amino acids catabolism. Dysregulation of FAO and an increase in plasma acylcarnitines are recognized as important in the pathophysiology of IR and T2DM. To investigate the deficiency of ACAD10 as a monogenic risk factor for T2DM in human, an Acad-deficient mouse was generated and characterized. The deficient mice exhibit an abnormal glucose tolerance test and elevated insulin levels. Blood acylcarnitine analysis shows an increase in long-chain species in the older mice. Nonspecific variable pattern of elevated short-terminal branch-chain acylcarnitines in a variety of tissues was also observed. Acad10 mice accumulate excess abdominal adipose tissue, develop an early inflammatory liver process, exhibit fasting rhabdomyolysis, and have abnormal skeletal muscle mitochondria. Our results identify Acad10 as a genetic determinant of T2DM in mice and provide a model to further investigate genetic determinants for insulin resistance in humans.



Similar content being viewed by others
Abbreviations
- ACADs:
-
Acyl-CoA dehydrogenases
- ACAD10:
-
Acyl-CoA dehydrogenase 10
- ACNs:
-
Acylcarnitines
- BN-PAGE:
-
Blue native polyacrylamide gel electrophoresis°
- GWAS:
-
Genome-wide association study
- T2DM:
-
Type 2 diabetes mellitus
- FAO:
-
FAO
References
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139(6):1073–1081
Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPhersonR DR, Hwang DH, Adams SH, Harper ME (2015) Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J 29(1):336–345
Baier LJ, Hanson RL (2004) Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes 53(5):1181–1186
Bennett PH (1999) Type 2 diabetes among the Pima Indians of Arizona: an epidemic attributable to environmental change? Nutr Rev 57(5 Pt 2):S51–S54
Bian L, Hanson RL, Muller YL, Ma L, Kobes S, Knowler WC, Bogardus C, Baier LJ (2010) Variants in ACAD10 are associated with type 2 diabetes, insulin resistance and lipid oxidation in Pima Indians. Diabetologia 53(7):1349–1353
Goodpaster BH (2013) Mitochondrial deficiency is associated with insulin resistance. Diabetes 62(4):1032–1035
Graves JA, Wang Y, Sims-Lucas S, Cherok E, Rothermund K, Branca MF, Elster J, Beer-Stolz D, Van Houten B, Vockley J, Prochownik EV (2012) Mitochondrial structure, function and dynamics are temporally controlled by c-Myc. PLoS One 7(5):e37699
He M, Pei Z, Mohsen A-W, Watkins P, Murdoch G, Van Veldhoven PP, Ensenauer R, Vockley J (2011) Identification and characterization of new long chain Acyl-CoA dehydrogenases. Mol Genet Metab 102(4):418–429
Houten SM, Wanders RJ (2010) A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J Inherit Metab Dis 33(5):469–477
Kelley DE, Goodpaster B, Wing RR, Simoneau J-A (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277(6 Pt 1):E1130–E1141
Lebovitz HE, Banerji MA (2005) Point: visceral adiposity is causally related to insulin resistance. Diabetes Care 28(9):2322–2325
Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, Knowler WC, Bennett PH, Bogardus C (1993) Insulin resistance and insulin secretory dysfunction as precursors of noninsulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 329(27):1988–1992
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307(5708):384–387
Michaliszyn SF, Sjaarda LA, Mihalik SJ, Lee S, Bacha F, Chace DH, De Jesus VR, Vockley J, Arslanian SA (2012) Metabolomic profiling of amino acids and beta-cell function relative to insulin sensitivity in youth. J Clin Endocrinol Metab 97(11):E2119–E2124
Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FGS, DeLany JP (2010) Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity 18(9):1695–1700
Mihalik SJ, Michaliszyn SF, de las Heras J, Bacha F, Lee S, Chace DH, DeJesus VR, Vockley J, Arslanian SA (2012) Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 35(3):605–611
Palladino AA, Chen J, Kallish S, Stanley CA, Bennett MJ (2012) Measurement of tissue acyl-CoAs using flow-injection tandem mass spectrometry: acyl-CoA profiles in short-chain FAO defects. Mol Genet Metab 107(4):679–683
Pociask DA, Scheller EV, Mandalapu S, McHugh KJ, Enelow RI, Fattman CL, Kolls JK, Alcorn F (2013) IL-22 is essential for lung epithelial repair following influenza infection. Am J Pathol 182(4):1286–1296
Pratley RE (1998) Gene-environment interactions in the pathogenesis of type 2 diabetes mellitus: lessons learned from the Pima Indians. Proc Nutr Soc 57(2):175–181
Prochazka M, Lillioja S, Tait JF, Knowler WC, Mott DM, Spraul M, Bennett PH, Bogardus C (1993) Linkage of chromosomal markers on 4q with a putative gene determining maximal insulin action in Pima Indians. Diabetes 42(4):514–519
Rehman KK, Wang Z, Bottino R, Balamurugan AN, Trucco M, Li J, Xiao X, Robbins PD (2005) Efficient gene delivery to human and rodent islets with double-stranded (ds) AAV-based vectors. Gene Ther 12(17):1313–1323
Scalco RS, Gardiner AR, Pitceathly RD, Zanoteli E, Becker J, Holton JL, Houlden H, Jungbluth H, Quinlivan R (2015) Rhabdomyolysis: a genetic perspective. Orphanet J Rare Dis 10(1):51
Schulz LO, Bennett PH, Ravussin E, Kidd JR, Kidd KK, Esparza J, Valencia ME (2006) Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care 29:1866–1871
Swigoňová Z, Mohsen A-W, Vockley J (2009) Acyl-CoA dehydrogenases: dynamic history of protein family evolution. J Mol Evol 69(2):176–193
Valencia ME, Bennett PH, Ravussin E, Esparza J, Fox C, Schulz LO (1999) The Pima Indians in Sonora, Mexico. Nutr Rev 57(5 Pt 2):S55–S57, S57-58
Van Coster R, Smet J, George E, De Meirleir L, Seneca S, Van Hove J, Sebire G, Verhelst H, De Bleecker J, Van Vlem B et al (2001) Blue native polyacrylamide gel electrophoresis: a powerful tool in diagnosis of oxidative phosphorylation defects. Pediatr Res 50(5):658–665
Vockley J, Marsden D, McCracken E, DeWard S, Barone A, Hsu K, Kakkis E (2015) Long-term major clinical outcomes in patients with long chain FAO disorders before and after transition to triheptanoin treatment-A retrospective chart review. Mol Genet Metab 116(1-2):53–60
Weiss R, Dufour S, Taksali SE, Tambortlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Alle K, Rife F et al (2003) Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362(9388):951–957
Acknowledgements
Jonathan Franks and Ming Sun at the University of Pittsburgh’s Center for Biologic Imaging provided expertise in transmission electron microscopy. This work was supported in part by PHS Grant R01-DK 54936. All mouse experiments were approved by the University of Pittsburgh Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Additional information
Communicated by: Eva Morava
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 39 kb)
Supplemental Fig. 1
A. In a 5-week period, mouse, food levels from 6–7 mice per group were weighed weekly using a standard lab balance. All wild-type and Acad10-deficient mice were approximately 8 months old. The “WT pooled” and “Acad10-deficient pooled” categories include combined data from both male and female mice. There were no significant differences in the amount of food consumed by wild-type mice (any group) compared with Acad10-deficient mice (any group). B. Zones of movement quantitated in open-field testing. To examine locomotor function and behaviors of mutant mice in a new environment, animals were placed for 30 min in an open-field testing chamber (28 x 28 x 40 cm) with a floor divided into equal-sized square fields, and the frequency of a variety of behaviors was determined. The lighting was set to 15–20 lux, similar to standard housing. The animal was removed from its home cage and placed into one of the four corners of the open-field chamber and allowed to explore the chamber freely for 30 min, following which, it was returned to its home cage. Testing parameters included (1) ambulatory distance, (2) velocity, (3) real vertical time, (4) real stereotypic time, (5) distance per trip, (6) real resting time, (7) real ambulatory time, (8) ambulatory counts, (9) stereotypic counts, and (10) vertical counts. C. Distance traveled in each zone is depicted with wild-type animals in blue and ACAD10–/– deficient in red. The Acad10-deficient female mice show a statistically significant decrease in movement measures in Zone 1 (two-tailed p value=0.0004,0.0002, and 0.0003, respectively), indicated by an asterisk (*), compared with wild-type control mice of the same background. The Acad10-deficient female mice show statistically significant decrease in movement measures in Zone 0 across time (two-tailed p value=0.0230), indicated by an asterisk (*), compared with wild-type control mice of the same background. (PPTX 171 kb)
Supplemental Fig. 2
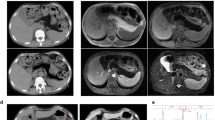
Mouse histopathology. A. Light microscopy with hematoxylin and eosin staining of wild-type liver (a) and ACAD10 –/– liver (b), and spleen (e) show the formation of small necroinflammatory lesions and early abscesses in liver, with extramedullary hematopoieisis and prominent megakaryocytes in spleen. B. Transmission electron microscopy of wild-type mouse liver (a) and muscle from hind quarter (c) was unremarkable. ACAD10-deficient liver (b) and muscle from hind quarter (d) show accumulation of lipid droplets (yellow arrows) and enlarged mitochondria (white arrow) in multiple representative sections. Muscle from hind quarter (d) also demonstrated abnormal shape and size of mitochondria in multiple representative sections. C. Specialized staining techniques of wild-type and deficient mouse tissues. a, b. Alkaline phosphatase staining shows peripheral nuclei indicative of chronic damage; c, d. Gomorri trichrome shows increased fat accumulation (purple) in deficient animals; e, f. Nicotinamide adenine dinucleotide, reduced (NADH) dehydrogenase staining shows an increased number of poorly staining muscle fibers. (PPTX 1049 kb)
Supplemental Fig. 3
Subcellular localization of ACAD10 varies in different tissues. A. Immunofluorescent staining of lung, pancreas, muscle, and kidney from wild-type mice with anitserum to ACAD10 (left column), the mitochondrial marker MTCO-1 (middle column), and the merged image (right column). All tissues showed colocalization of ACAD10 with the mitochondrial marker. B. In addition, lung and pancreas were stained with antibodies to the peroxisomal marker catalase (left column). Right column shows merged images, and identifies colocalization of ACAD10 to peroxisomes as well as mitochondria in lung and pancreas; ×60 all images. (PPTX 686 kb)
Supplemental Fig. 4
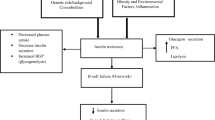
A. Hepatic phosphoenolpyruvate carboxykinase (PEPCK) expression and respiratory chain function. There is significant increase in hepatic expression of PEPCK in Acad10-deficient mice, which indicates hepatic insulin resistance. B. Blue native gel electrophoresis showing changes in expression of the complexes of the electron transport chain. Complex I activity is increased in liver, muscle, and brain. Complex V activity is increased in heart, liver, and muscle. (PPTX 196 kb)
Rights and permissions
About this article
Cite this article
Bloom, K., Mohsen, AW., Karunanidhi, A. et al. Investigating the link of ACAD10 deficiency to type 2 diabetes mellitus. J Inherit Metab Dis 41, 49–57 (2018). https://doi.org/10.1007/s10545-017-0013-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-017-0013-y