Abstract
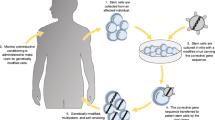
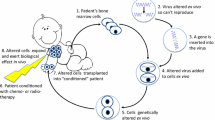
In the last years important progress has been made in the treatment of several primary immunodeficiency disorders (PIDs) with gene therapy. Hematopoietic stem cell (HSC) gene therapy indeed represents a valid alternative to conventional transplantation when a compatible donor is not available and recent success confirmed the great potential of this approach. First clinical trials performed with gamma retroviral vectors were promising and guaranteed clinical benefits to the patients. On the other hand, the outcome of severe adverse events as the development of hematological abnormalities highlighted the necessity to develop a safer platform to deliver the therapeutic gene. Self-inactivating (SIN) lentiviral vectors (LVVs) were studied to overcome this hurdle through their preferable integration pattern into the host genome. In this review, we describe the recent advancements achieved both in vitro and at preclinical level with LVVs for the treatment of Wiskott-Aldrich syndrome (WAS), chronic granulomatous disease (CGD), ADA deficiency (ADA-SCID), Artemis deficiency, RAG1/2 deficiency, X-linked severe combined immunodeficiency (γchain deficiency, SCIDX1), X-linked lymphoproliferative disease (XLP) and immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome.

Similar content being viewed by others
References
Aiuti A, Vai S, Mortellaro A et al (2002) Immune reconstitution in ADA-SCID after PBL gene therapy and discontinuation of enzyme replacement. Nat Med 8(5):423–425
Aiuti A, Cattaneo F, Galimberti S et al (2009) Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 360(5):447–458
Aiuti A, Bacchetta R, Seger R, Villa A, Cavazzana-Calvo M (2012) Gene therapy for primary immunodeficiencies: Part 2. Curr Opin Immunol 24(5):585–591
Aiuti A, Biasco L, Scaramuzza S et al (2013a) Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341(6148):1233151
Aiuti A, Cossu G, de Felipe P et al (2013b) The committee for advanced therapies' of the European Medicines Agency reflection paper on management of clinical risks deriving from insertional mutagenesis. Hum Gene Ther Clin Dev 24(2):47–54
Allan SE, Passerini L, Bacchetta R et al (2005) The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J Clin Invest 115(11):3276–3284
Allan SE, Alstad AN, Merindol N et al (2008) Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol Ther 16(1):194–202
Antoniou M, Harland L, Mustoe T et al (2003) Transgenes encompassing dual-promoter CpG islands from the human TBP and HNRPA2B1 loci are resistant to heterochromatin-mediated silencing. Genomics 82(3):269–279
Astrakhan A, Sather BD, Ryu BY et al (2012) Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood 119(19):4395–4407
Barde I, Laurenti E, Verp S et al (2011) Lineage- and stage-restricted lentiviral vectors for the gene therapy of chronic granulomatous disease. Gene Ther 18(11):1087–1097
Barzaghi F, Passerini L, Gambineri E et al (2012) Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J Autoimmun 38(1):49–58
Benjelloun F, Garrigue A, Demerens-de Chappedelaine C et al (2008) Stable and functional lymphoid reconstitution in artemis-deficient mice following lentiviral artemis gene transfer into hematopoietic stem cells. Mol Ther 16(8):1490–1499
Bianchi M, Hakkim A, Brinkmann V et al (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114(13):2619–2622
Biasco L, Ambrosi A, Pellin D et al (2011) Integration profile of retroviral vector in gene therapy treated patients is cell-specific according to gene expression and chromatin conformation of target cell. EMBO Mol Med 3(2):89–101
Biasco L, Baricordi C, Aiuti A (2012) Retroviral integrations in gene therapy trials. Mol Ther 20:709–716
Biffi A, Montini E, Lorioli L et al (2013) Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341(6148):1233158
Booth C, Gilmour KC, Veys P et al (2011) X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood 117(1):53–62
Bousfiha AA, Jeddane L, Ailal F et al (2013) A phenotypic approach for IUIS PID classification and diagnosis: guidelines for clinicians at the bedside. J Clin Immunol 33(6):1078–1087
Bousso P, Wahn V, Douagi I et al (2000) Diversity, functionality, and stability of the T cell repertoire derived in vivo from a single human T cell precursor. Proc Natl Acad Sci U S A 97(1):274–278
Boztug K, Schmidt M, Schwarzer A et al (2010) Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med 363(20):1918–1927
Brady T, Agosto LM, Malani N, Berry CC, O'Doherty U, Bushman F (2009) HIV integration site distributions in resting and activated CD4+ T cells infected in culture. AIDS 23(12):1461–1471
Brendel C, Muller-Kuller U, Schultze-Strasser S et al (2011) Physiological regulation of transgene expression by a lentiviral vector containing the A2UCOE linked to a myeloid promoter. Gene Ther 24(3):1018–1029
Brown BD, Naldini L (2009) Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 10(8):578–585
Brown BD, Gentner B, Cantore A et al (2007) Endogenous microRNA can be broadly exploited to regulate transgene expression according to tissue, lineage and differentiation state. Nat Biotechnol 25(12):1457–1467
Candotti F, Shaw KL, Muul L et al (2012) Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood 120(18):3635–3646
Carbonaro DA, Jin X, Cotoi D et al (2008) Neonatal bone marrow transplantation of ADA-deficient SCID mice results in immunologic reconstitution despite low levels of engraftment and an absence of selective donor T lymphoid expansion. Blood 111(12):5745–5754
Carbonaro DA, Zhang L, Jin X, et al (2013) Pre-clinical demonstration of lentiviral vector mediated correction of immunological and metabolic abnormalities in models of adenosine deaminase deficiency. Mol Ther doi:10.1038/mt.2013.265
Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G et al (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288(5466):669–672
Charrier S, Dupre L, Scaramuzza S et al (2007) Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther 14(5):415–428
Chinen J, Davis J, De Ravin SS et al (2007) Gene therapy improves immune function in preadolescents with X-linked severe combined immunodeficiency. Blood 110(1):67–73
Corrigan-Curay J, Cohen-Haguenauer O, O'Reilly M et al (2012) Challenges in vector and trial design using retroviral vectors for long-term gene correction in hematopoietic stem cell gene therapy. Mol Ther 20(6):1084–1094
Di Matteo G, Giordani L, Finocchi A et al (2009) Molecular characterization of a large cohort of patients with Chronic Granulomatous Disease and identification of novel CYBB mutations: an Italian multicenter study. Mol Immunol 46(10):1935–1941
Dupre L, Marangoni F, Scaramuzza S et al (2006) Efficacy of gene therapy for Wiskott-Aldrich syndrome using a WAS promoter/cDNA-containing lentiviral vector and nonlethal irradiation. Hum Gene Ther 17(3):303–313
Fischer A, Cavazzana-Calvo M (2008) Gene therapy of inherited diseases. Lancet 371(9629):2044–2047
Gaspar HB, Cooray S, Gilmour KC et al (2011a) Long-term persistence of a polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med 3(97):97ra79
Gaspar HB, Cooray S, Gilmour KC et al (2011b) Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci Transl Med 3(97):97ra80
Gennery AR, Slatter MA, Grandin L et al (2010) Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol 126(3):602–610, e601-611
Greene MR, Lockey T, Mehta PK et al (2012) Transduction of human CD34+ repopulating cells with a self-inactivating lentiviral vector for SCID-X1 produced at clinical scale by a stable cell line. Hum Gene Ther Methods 23(5):297–308
Grez M, Reichenbach J, Schwable J, Seger R, Dinauer MC, Thrasher AJ (2011) Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther 19(1):28–35
Griffith LM, Cowan MJ, Notarangelo LD et al (2013) Primary Immune Deficiency Treatment Consortium (PIDTC) report. J Allergy Clin Immunol doi:10.1016/j.jaci.2013.07.052.
Gungor T, Teira P, Slatter M et al (2014) Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet 383(9915):436–448
Hacein-Bey-Abina S, von Kalle C, Schmidt M et al (2003) A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 348(3):255–256
Hacein-Bey-Abina S, Hauer J, Lim A et al (2010) Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 363(4):355–364
Hassan A, Booth C, Brightwell A et al (2012) Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood 120(17):3615–3624, quiz 3626
Henter JI, Samuelsson-Horne A, Arico M et al (2002) Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood 100(7):2367–2373
Hershfield MS (1998) Adenosine deaminase deficiency: clinical expression, molecular basis, and therapy. Semin Hematol 35(4):291–298
Holland SM (2013) Chronic granulomatous disease. Hematol Oncol Clin North Am 27(1):89–99, viii. doi:10.1016/j.hoc.2012.11.002
Huston MW, van Til NP, Visser TP et al (2011) Correction of murine SCID-X1 by lentiviral gene therapy using a codon-optimized IL2RG gene and minimal pretransplant conditioning. Mol Ther 19(10):1867–1877
Kang EM, Marciano BE, DeRavin S, Zarember KA, Holland SM, Malech HL (2011) Chronic granulomatous disease: overview and hematopoietic stem cell transplantation. J Allergy Clin Immunol 127(6):1319–1326, quiz 1327-1318
Kaufmann KB, Buning H, Galy A, Schambach A, Grez M (2013) Gene therapy on the move. EMBO Mol Med 5(11):1642–1661
Lagresle-Peyrou C, Benjelloun F, Hue C et al (2008) Restoration of human B-cell differentiation into NOD-SCID mice engrafted with gene-corrected CD34+ cells isolated from Artemis or RAG1-deficient patients. Mol Ther 16(2):396–403
Ma CS, Nichols KE, Tangye SG (2007) Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol 25:337–379
Marangoni F, Poli A, Agostoni C et al (2009) A consensus document on the role of breakfast in the attainment and maintenance of health and wellness. Acta Biomed 80(2):166–171
Massaad MJ, Ramesh N, Geha RS (2013) Wiskott-Aldrich syndrome: a comprehensive review. Ann N Y Acad Sci 1285:26–43
Moratto D, Giliani S, Bonfim C et al (2011) Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood 118(6):1675–1684
Mortellaro A, Hernandez RJ, Guerrini MM et al (2006) Ex vivo gene therapy with lentiviral vectors rescues adenosine deaminase (ADA)-deficient mice and corrects their immune and metabolic defects. Blood 108(9):2979–2988
Moshous D, Callebaut I, de Chasseval R et al (2001) Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105(2):177–186
Mukherjee S, Thrasher AJ (2013) Gene therapy for PIDs: progress, pitfalls and prospects. Gene 525(2):174–181
Multhaup M, Karlen AD, Swanson DL et al (2010) Cytotoxicity associated with artemis overexpression after lentiviral vector-mediated gene transfer. Hum Gene Ther 21(7):865–875
Neven B, Leroy S, Decaluwe H et al (2009) Long-term outcome after hematopoietic stem cell transplantation of a single-center cohort of 90 patients with severe combined immunodeficiency. Blood 113(17):4114–4124
Noguchi M, Yi H, Rosenblatt HM et al (1993) Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73(1):147–157
Ott MG, Schmidt M, Schwarzwaelder K et al (2006) Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med 12(4):401–409
Pachlopnik Schmid J, Canioni D, Moshous D et al (2011) Clinical similarities and differences of patients with X-linked lymphoproliferative syndrome type 1 (XLP-1/SAP deficiency) versus type 2 (XLP-2/XIAP deficiency). Blood 117(5):1522–1529
Pai SY, Notarangelo LD (2010) Hematopoietic cell transplantation for Wiskott-Aldrich syndrome: advances in biology and future directions for treatment. Immunol Allergy Clin North Am 30(2):179–194
Passerini L, Mel ER, Sartirana C et al (2013) CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci Transl Med 5(215):215ra174
Pike-Overzet K, Rodijk M, Ng YY et al (2011) Correction of murine Rag1 deficiency by self-inactivating lentiviral vector-mediated gene transfer. Leukemia 25(9):1471–1483
Revy P, Buck D, le Deist F, de Villartay JP (2005) The repair of DNA damages/modifications during the maturation of the immune system: lessons from human primary immunodeficiency disorders and animal models. Adv Immunol 87:237–295
Rivat C, Booth C, Alonso-Ferrero M et al (2013) SAP gene transfer restores cellular and humoral immune function in a murine model of X-linked lymphoproliferative disease. Blood 121(7):1073–1076
Santilli G, Almarza E, Brendel C et al (2011) Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells. Mol Ther 19(1):122–132
Scaramuzza S, Biasco L, Ripamonti A et al (2013) Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome. Mol Ther 21(1):175–184
Schwarz K, Gauss GH, Ludwig L et al (1996) RAG mutations in human B cell-negative SCID. Science 274(5284):97–99
Scobie L, Hector RD, Grant L et al (2009) A novel model of SCID-X1 reconstitution reveals predisposition to retrovirus-induced lymphoma but no evidence of gammaC gene oncogenicity. Mol Ther 17(6):1031–1038
Seger RA (2010) Advances in the diagnosis and treatment of chronic granulomatous disease. Curr Opin Hematol Nov 11. [Epub ahead of print]
Stein S, Ott MG, Schultze-Strasser S et al (2010) Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat Med 16(2):198–204
Thrasher AJ, Burns SO (2010) WASP: a key immunological multitasker. Nat Rev Immunol 10(3):182–192
van der Loo JC, Swaney WP, Grassman E et al (2012) Critical variables affecting clinical-grade production of the self-inactivating gamma-retroviral vector for the treatment of X-linked severe combined immunodeficiency. Gene Ther 19(8):872–876
Winkelstein JA, Marino MC, Johnston RB Jr et al (2000) Chronic granulomatous disease. Report on a national registry of 368 patients. Med (Baltimore) 79(3):155–169
Yagi H, Nomura T, Nakamura K et al (2004) Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol 16(11):1643–1656
Zhang F, Thornhill SI, Howe SJ et al (2007) Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood 110(5):1448–1457
Zhang F, Frost AR, Blundell MP, Bales O, Antoniou MN, Thrasher AJ (2010) A ubiquitous chromatin opening element (UCOE) confers resistance to DNA methylation-mediated silencing of lentiviral vectors. Mol Ther 18(9):1640–1649
Zhou S, Mody D, DeRavin SS et al (2010) A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells. Blood 116(6):900–908
Ziegler SF (2006) FOXP3: of mice and men. Annu Rev Immunol 24:209–226
Acknowledgments
This work was supported by grants from Fondazione Roma (Stem Cells based approaches to monogenic diseases); European Commission (E-rare project EURO-CGD and CELL-PID HEALTH-F5-2010-261387) and Fondazione Telethon (TIGET core grant).
Compliance with ethics guidelines
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Jean-Marie Saudubray
Giada Farinelli and Valentina Capo contributed equally to this work
Rights and permissions
About this article
Cite this article
Farinelli, G., Capo, V., Scaramuzza, S. et al. Lentiviral vectors for the treatment of primary immunodeficiencies. J Inherit Metab Dis 37, 525–533 (2014). https://doi.org/10.1007/s10545-014-9690-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-014-9690-y