Abstract
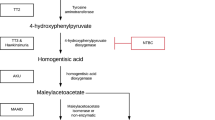
NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3cyclohexanedione) is the mainstay of treatment in tyrosinemia type 1 (HT 1). The current recommendation is to divide the total daily dose of NTBC into two doses. We monitored the plasma NTBC concentrations in a series of seven patients who were changed from multiple divided doses to a single daily dose of NTBC. Two additional patients were started on a single daily dose of NTBC after the diagnosis of HT 1 was established. In three patients, NTBC kinetics were performed over 6 and 24 hours, respectively. The use of multiple divided doses or a single daily dose did not significantly affect plasma NTBC concentrations or the mean daily dose needed to attain therapeutic plasma NTBC concentrations. Moreover, kinetic studies demonstrated that plasma NTBC concentrations were completely stable over a period of 24 hours with a single dose regimen, as expected given the known NTBC plasma half life of 54 hours. Although these preliminary results need to be confirmed in more patients, our findings show that administration of NTBC in a single daily dose may be as effective as a multiple-dose regimen in reaching therapeutic plasma NTBC concentrations and suppressing succinylacetone formation in patients with HT 1. In fact, single dose treatment may increase patients’ compliance with the drug treatment and improve metabolic control.




Similar content being viewed by others
References
Chapkrani A, Holme E (2006) Disorders of tyrosine metabolism. In: Fernandes J, Saudubray JM, Van Den Berghe G, Walter JH (eds) Inborn metabolic diseases, 4th edn. Springer, Berlin, pp 233–243
D’Eufemia P, Celli M, Tetti M et al (2011) Tyrosinemia type I: long-term outcome in a patient treated with doses of NTBC lower than recommended. Eur J Pediatr 170:819
El-Karaksy H, Rashed M, El-Sayed R et al (2010) NTBC therapy for tyrosinemia type 1: how much is enough? Eur J Pediatr 169:689–693
Hall MG, Wilks MF, McLean Provan W et al (2001) Pharmacokinetics and pharmacodynamics of NTBC (2-(2-nitro-4-fluoromethylbenzoyl)-1,3-cyclohexanedione) and mesotrione, inhibitors of 4-hydroxyphenyl pyruvate dioxygenase (HPPD) following a single dose to healthy male volunteers. Br J Clin Pharmacol 52:169–177
Herebian D, Spiekerkötter U, Lamshöft M et al (2009) Liquid chromatography tandem mass spectrometry method for the quantitation of NTBC (2-(nitro-4-trifluoromethylbenzoyl)1,3-cyclohexanedione) in plasma of tyrosinemia type 1 patients. J Chromatogr B 877:1453–1459
Holme E (2006) Disorders of tyrosine degradation. In: Blau N, Leonard J, Hoffmann GF, Clarke JTR (eds) Physicians Guide to the Treatment and Follow-Up of Metabolic Diseases. Springer, Berlin, pp 49–56
Holme E, Lindstedt S (1995) Diagnosis and management of tyrosinemia type 1. Curr Opin Pediatr 7:726–732
Holme E, Lindstedt S (1998) Tyrosinemia type 1 and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J Inherit Metab Dis 21:507–517
Holme E, Lindstedt S (2000) Nontransplant treatment of tyrosinemia. Clin Liver Dis 4:805–814
Jorquera R, Tanguay RM (2001) Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 10:1741–1752
Joshi SN, Venugopalan P (2004) Experience with NTBC therapy in hereditary tyrosinemia type 1: an alternative to liver transplantation. Anal Trop Paediatr 24:1741–1752
Masurel-Paulet A, Poggi-Bach J, Rolland MO et al (2008) NTBC treatment in tyrosinaemia type I: Long-term outcome in French patients. J Inherit Metab Dis 31:81–87
Mitchell G, Grompe M, Lambert M et al (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw-Hill, New York, pp 1777–1805
Orfadin [EPAR product information]. European Medicines Agency, London; Retrieved from http://www.ema.europa.eu/docs/en-GB/document_library/EPAR_-_Product_Information/human/000555/WC500049195.pdf. Accessed Oct 31, 2011.
Orfadin [package insert]. Swedish Orphan Biovitrium International AB, Sweden; September 2010. Retrieved from http://orfadin.com/orfadin_pkg_insert.pdf. Accessed 31 Oct 2011
Prieto JA, Andrade F, Lage L et al (2011) Comparison of plasma and dry blood spots as samples for the determination of nitisinone (NTBC) by high-performance liquid chromatography-tandem mass spectrometry. Study of the stability of the samples at different temperatures. J Chromatogr B. doi:10.1016/j.jchromb.2011.01.31
Russo P, O’Regan S (1990) Visceral pathology of hereditary tyrosinemia type I. Am J Hum Genet 47:317–324
Sander J, Janzen N, Terhardt M et al (2011) Monitoring tyrosinemia type I: Blood spot test for nitisinone (NTBC). Clin Chim Acta 412:134–138
Schlump JU, Perot C, Ketteler K et al (2008) Severe neurological crisis in a patient with hereditary tyrosinemia type I after interruption of NTBC. J Inherit Metab Dis, published online.
Thimm E, Herebian D, Assmann B et al (2011) Increase of CSF tyrosine and impaired serotonin turnover in tyrosinemia type I. Mol Genet Metab 102:122–125
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Matthias Baumgartner
Competing interest: None declared.
Rights and permissions
About this article
Cite this article
Schlune, A., Thimm, E., Herebian, D. et al. Single dose NTBC-treatment of hereditary tyrosinemia type I. J Inherit Metab Dis 35, 831–836 (2012). https://doi.org/10.1007/s10545-012-9450-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-012-9450-9