Summary
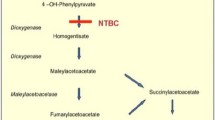
We describe a retrospective study of long-term outcome of 46 patients treated and regularly followed in France with 2-(2-nitro-4-trifluoromethylbenzoyl)-1, 3-cyclohexanedione (NTBC) for tyrosinaemia type I. Most had initial good response with normalization of liver function and metabolic parameters. Only one infant had no response to treatment and required liver transplantation. Among the 45 long-term treated patients, three underwent secondary liver transplantation: one for cirrhosis and two because of hepatocellular carcinoma. One of the latter died of transplantation complications, so that the overall survival rate was 97.5%. However, 17 of 45 showed persistent abnormal liver imaging (heterogeneous liver) and 6 had cirrhosis. Furthermore, 15 had persistently elevated levels of alpha-fetoprotein, highlighting the question of the persistent risk of carcinoma. Quality of life was usually good but compliance problems were frequent, mainly regarding the low phenylalanine–tyrosine diet. Few adverse effects were observed. A main concern was the high frequency of cognitive impairment causing schooling problems, which may be related to persistent chronic hypertyrosinaemia. In conclusion, this series confirms that NTBC treatment has clearly improved the vital prognosis and quality of life of tyrosinaemia type I patients but that many late complications persist. Long-term studies are necessary to determine whether this drug may prevent or only delay liver complications, andto survey the possible risks of the drug. A more restricted diet could be necessary to prevent the neurological impact of the disease.
Similar content being viewed by others
References
Al-Dhalamy M, Overturf K, Finegold M, Grompe M (2002) Long-term therapy with NTBC and tyrosine-restricted diet in a murine model of hereditary tyrosinemia type I. Mol Genet Metab 75: 38–45.
Baumann U, Duhme, Auth MKH, et al (2006) Lectin-reactive alpha-Fetoprotein in patients with Tyrosinaemia Type 1 and Hepatocellular Carcinoma. J Pediatr Gastroenterol Nutr 43: 77–82.
Cerone R, Holme E, Schiaffino MC, et al (1997) Tyrosinemia type III: diagnosis and ten-year follow-up. Acta Paediatr 86: 1013–1015.
Chakrapani A, Holme E (2006) Disorders of tyrosine metabolism. In: Fernandes J, Saudubray JM, Van Den Berghe G, Walter JH eds. Inborn Metabolic Diseases, 4th edn. Berlin: Springer Medizin Verlag, 233–243.
Crone J, Möslinger D, Bodamer OA, et al (2003) Reversibility of cirrhotic regenerative liver nodules upon NTBC treatment in a child with tyrosinemia type I. Acta Paediatr 92: 625–628.
Ellaway CJ, Holme E, Standing S (2001) Outcome of tyrosinemia type III. J Inherit Metab Dis 24: 824–832.
Forget S, Patriquin HB, Dubois J, et al (1999) The kidney in children with tyrosinemia: sonographic, CT and biochemical findings. Pediatr Radiol 29: 104–108.
Holme E, Lindstedt S (1995) Diagnosis and management of tyrosinemia type 1. Curr Opin Pediatr 7: 726–732.
Holme E, Lindstedt S (1998) Tyrosinaemia type 1 and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J Inherit Metab Dis 21: 507–517.
Holme E, Lindstedt S (2000) Nontransplant treatment of tyrosinemia. Clin Liver Dis 4: 805–814.
Jacobs SM, van Beurden DH, Klomp LW, et al (2006) Kidneys of mice with hereditary tyrosinemia typa 1 are extremely sensitive to cytotoxicity. Pediatr Res 59: 365–370.
Jorquera R, Tanguay RM (2001) Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 10: 1741–1752.
Joshi SN, Venugopalan P (2004) Experience with NTBC therapy in hereditary tyrosinaemia type 1: an alternative to liver transplantation. Anal Trop Paediatr 24: 259–265.
Lindstedt S, Holme E, Lock EA, et al (1992) Treatment of hereditary tyrosinaemia type 1 by inhibition of 4-hydroxyphanylpyruvate dioxygenase. Lancet 340: 813–817.
Lock EA, Ellis MK, Gaskin P, et al (1998) From toxicological problem to therapeutic use: The discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 21: 498–506.
Mitchell G, Larochelle J, Lambert M (1990) Neurologic crises in hereditary tyrosinemia. N Engl J Med 322: 432–437.
Mitchell G, Grompe M, Lambert M, et al (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds; Childs B, Kinzler KW, Vogelstein B, assoc, eds. The Metabolic and Molecular Bases of Inherited Disease, 8th edn. New York: McGraw-Hill, 1777–1805.
Mohan N, McKiernan P, Preece MA (1999) Indications and outcome of liver transplantation in tyrosinemia type 1. Eur J Pediatr 158: S49–S54.
Russo P, O’Reagan S (1990) Visceral pathology of hereditary tyrosinemia type 1. Am J Hum Genet 47: 317–324.
Van Spronsen FJ, Thomasse Y, Smit GPA, et al (1994) Hereditary tyrosinemia type 1: a new clinical classification with difference in prognosis on dietary treatment. Hepatology 5: 1187–1190.
Van Spronsen FJ, Smit GPA, Wijburg FA, et al (1995) Tyrosinemia type 1: considerations of treatment strategy and experiences with risk assessment, diet and transplantation. J Inherit Metab Dis 18: 111–114.
Van Spronsen FJ, Bijleveld CMA, van Maldegem BT, et al (2005) Hepatocellular carcinoma in hereditary tyrosinemia type 1 despite 2-(2-nitro-4,3-trifluoro-methylbenzoyl)-1,3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr 40: 90–93.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: Markus Grompe
Competing interests: None declared
References to electronic databases: Tyrosinaemia type I, OMIM 276700. Fumarylacetoacetase, EC 3.7.1.2.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Individual data of the 46 long-term treated patients mos = months of age; Tt = NTBC treatment; ANL = Abnormal; Nl = Normal; N = No; Y = Yes (DOC 112 kb)
Rights and permissions
About this article
Cite this article
Masurel-Paulet, A., Poggi-Bach, J., Rolland, MO. et al. NTBC treatment in tyrosinaemia type I: Long-term outcome in French patients. J Inherit Metab Dis 31, 81–87 (2008). https://doi.org/10.1007/s10545-008-0793-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0793-1