Abstract
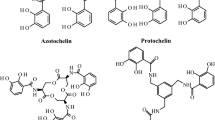
Although siderophores are generally viewed as biological iron uptake agents, recent evidence has shown that they may play significant roles in the biogeochemical cycling and biological uptake of other metals. One such siderophore that is produced by A. vinelandii is the triscatecholate protochelin. In this study, we probe the solution chemistry of protochelin and its complexes with environmentally relevant trace metals to better understand its effect on metal uptake and cycling. Protochelin exhibits low solubility below pH 7.5 and degrades gradually in solution. Electrochemical measurements of protochelin and metal–protochelin complexes reveal a ligand half-wave potential of 200 mV. The Fe(III)Proto3− complex exhibits a salicylate shift in coordination mode at circumneutral to acidic pH. Coordination of Mn(II) by protochelin above pH 8.0 promotes gradual air oxidation of the metal center to Mn(III), which accelerates at higher pH values. The Mn(III)Proto3− complex was found to have a stability constant of log β110 = 41.6. Structural parameters derived from spectroscopic measurements and quantum mechanical calculations provide insights into the stability of the Fe(III)Proto3−, Fe(III)H3Proto, and Mn(III)Proto3− complexes. Complexation of Co(II) by protochelin results in redox cycling of Co, accompanied by accelerated degradation of the ligand at all solution pH values. These results are discussed in terms of the role of catecholate siderophores in environmental trace metal cycling and intracellular metal release.








Similar content being viewed by others
References
Abergel RJ, Warner JA, Shuh DK, Raymond KN (2006) Enterobactin protonation and iron release: structural characterization of the salicylate coordination shift in ferric enterobactin. J Am Chem Soc J128:8920–8931
Abergel RJ, Zawadzka AM, Hoette TM, Raymond KN (2009) Enzymatic hydrolysis of trilactone siderophores: where chiral recognition occurs in enterobactin and bacillibactin iron transport. J Am Chem Soc 131(35):12682–12692
Ahrland S, Dahlgren A, Persson I (1990) Stabilities and hydrolysis of some iron(II) and manganese(III) complexes with chelating ligands. Acta Agric Scand 40:101–111
Albrecht-Gary AM, Crumbliss AL (1998) Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. In: Sigel A, Sigel H (eds) Metal ions in biological systems. M. Dekker, New York
Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM (2000) Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes. Anal Chem 72(24):5994–6002
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Bellenger JP, Arnaud-Neu F, Asfari Z, Myneni SCB, Stiefel EI, Kraepiel AML (2007) Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J Biol Inorg Chem 12(3):367–376. doi:10.1007/s00775-006-0194-6
Bellenger JP, Wichard T, Kraepiel AML (2008a) Vanadium requirements and uptake kinetics in the dinitrogen-fixing bacterium Azotobacter vinelandii. Appl Environ Microbiol 74(5):1478–1484. doi:10.1128/aem.02236-07
Bellenger JP, Wichard T, Kustka AB, Kraepiel AML (2008b) Uptake of molybdenum and vanadium by a nitrogen-fixing soil bacterium using siderophores. Nat Geosci 1(4):243–246. doi:10.1038/Ngeo161
Bellenger JP, Wichard T, Xu Y, Kraepiel AML (2011) Essential metals for nitrogen fixation in a free-living N2-fixing bacterium: chelation, homeostasis and high use efficiency. Environ Microbiol 13(6):1395–1411. doi:10.1111/j.1462-2920.2011.02440.x
Bodini M, Osorio C, Valle MAd, Aranciba V, Munoz G (1995) Redox chemistry of 3,4-dihydroxy-2-benzoic acid, its oxidation products and their interaction with manganese(II) and manganese(III). Polyhedron 14(20–21):2933–2936
Boukhalfa H, Reilly SD, Smith WH, Neu MP (2004) EDTA and mixed-ligand complexes of tetravalent and trivalent plutonium. Inorg Chem 43:5816
Boukhalfa H, Reilly SD, Neu MP (2007) Complexation of Pu(IV) with the natural siderophore desferrioxamine B and the redox properties of Pu(IV)(siderophore) complexes. Inorg Chem 46:1018
Buerge IJ, Hug SJ (1998) Influence of organic ligands on chromium(VI) reduction by iron(II). Environ Sci Technol 32:2092–2099
Carrano CJ, Drechsel H, Kaiser D, Jung G, Matzanke B, Winkelmann G, Rochel N, Albrecht-Gary AM (1996) Coordination chemistry of the carboxylate type siderophore rhizoferrin: the iron(III) complex and its metal analogs. Inorg Chem 35:6429–6436
Clarke ET, Tari LW, Vogel HJ (2001) Structural biology of bacterial iron uptake systems. Curr Top Med Chem 1:7
Cornish AS, Page WJ (1995) Production of the Triscatecholate Siderophore Protochelin by Azotobacter vinelandii. Biometals 8(4):332–338
Cornish AS, Page WJ (2000) Role of molybdate and other transition metals in the accumulation of protochelin by Azotobacter vinelandii. Appl Environ Microbiol 66(4):1580–1586
Crumbliss AL, Harrington JM (2009) Iron sequestration by small molecules: thermodynamic and kinetic studies of natural siderophores and synthetic model compounds. In: van Eldik R (ed) Advance in inorganic chemistry, vol 61. Elsevier, Amsterdam, pp 179–250
Dahlheimer SR, Neal CR, Fein JB (2007) Potential mobilization of platinum-group elements by siderophores in surface environments. Environ Sci Technol 41(3):870–875
Devlin HR, Harris IJ (1984) Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Ind Eng Chem Fundam 23:387–392
Downward L, Booth CH, Lukens WW, Bridges FA (2006) Variation of the F-test for determining statistical relevance of particular parameters in EXAFS fits. In: Hedman B, Pianetta P (eds) 13th international conference on X-Ray absorption fine structure. Stanford Synchrotron Radiation Laboratory, Stanford
Duckworth OW, Sposito G (2005) Siderophore–manganese(III) interactions I. Air-oxidation of manganese(II) promoted by desferrioxamine B. Environ Sci Technol 39:6037–6044
Duckworth OW, Bargar JR, Sposito G (2008) Sorption of ferric iron from ferrioxamine B to synthetic and biogenic layer type manganese oxides. Geochim Cosmochim Acta 72:3371–3380
Duckworth OW, Bargar JR, Sposito G (2009a) Quantitative structure–activity relationships for aqueous metal–siderophore complexes. Environ Sci Technol 43:343–349
Duckworth OW, Holmström SJM, Pena J, Sposito G (2009b) Biogeochemistry of iron oxidation in a circumneutral freshwater habitat. Chem Geol 260(3–4):149–158
Duckworth OW, Jarzecki AA, Bargar JR, Oyerinde O, Spiro TG, Sposito G (2009c) An exceptionally stable cobalt(III)–desferrioxamine B complex. Mar Chem 113:114–122
Duhme AK, Hider RC, Khodr HH (1997) Synthesis and iron-binding properties of protochelin, the tris(catecholamide) siderophore of Azotobacter vinelandii. Chem Ber Recueil 130:969–973
Duhme AK, Hider RC, Naldrett MJ, Pau RN (1998) The stability of the molybdenum–azotochelin complex and its effect on siderophore production in Azotobacter vinelandii. J Biol Inorg Chem 3(5):520–526
Essén SA, Bylund D, Holmström SJA (2006) Quantification of hydroxamate siderophores in soil solutions of podzolic soil profiles in Sweden. Biometals 19(3):269–282
Frazier SW, Kretzschmar K, Kraemer SM (2005) Bacterial siderophores promote dissolution of UO2 under reducing conditions. Environ Sci Technol 39(15):5709–5715
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, A J, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Daniels S, Dapprich AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. B, 1st edn. Gaussian Inc., Wallingford
Gans P, Sabatini A, Vacca A (1996) Investigation of equilibria in solution. Determination of equilibrium constants with HYPERQUAD suite of programs. Talanta 43(10):1739–1753
Hamilton WC (1965) Significance tests on the crystallographic R factor. Acta Crystallogr 18:502–510
Harrington JM, Park H, Ying Y, Hong J, Crumbliss AL (2011a) Characterization of Fe(III) sequestration by an analog of the cytotoxic siderophore brasilibactin A: implications for the iron transport mechanism in mycobacteria. Metallomics 3:464–471
Harrington JM, Parker DL, Jarzecki AA, Bargar JR, Tebo BM, Duckworth OW (2011b) Structural dependence of manganese chelation by siderophores: Donor group dependence of complex stability and reactivity. Geochim Cosmochim Acta (inpress)
Harris WR, Carrano CJ, Raymond KN (1979a) Coordination chemistry of microbial iron transport compounds. 16. Isolation, characterization, and formation constants of ferric aerobactin. J Am Chem Soc 101:2722
Harris WR, Carrano CR, Cooper SR, Sofen SR, Avdeef AE, McArdle JV, Raymond KN (1979b) Coordination chemistry of microbial iron transport compounds. 19. Stability constants and electrochemical behavior of ferric enterobactin and model complexes. J Am Chem Soc 101:6097–6104
Harris WR, Amin SA, Kuepper FC, Green DH, Carrano CJ (2007) Borate binding to siderophores: structure and stability. J Am Chem Soc 129:12263
Hay BP, Dixon DA, Vargas R, Garza J, Raymond KN (2001) Structural criteria for the rational design of selective ligands. 3. Quantitative structure–stability relationship for Iron(III) complexation by tris-catecholamide siderophores. Inorg Chem 40:3922–3935
Heien M, Johnson MA, Wightman RM (2004) Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem 76(19):5697–5704. doi:10.1021/ac0491509
Hernlem BJ, Vane LM, Sayles GD (1999) The application of siderophores for metal recovery and waste remediation: examination of correlations for prediction of metal affinities. Water Resour 33:951–960
Hewkin DJ, Prince RH (1970) The mechanism of octahedral complex formation by labile metal ions. Coord Chem Rev 5:45–73
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27:637–657
Holmström SJA, Lundstrom US, Finlay RD, Van Hees PAW (2004) Siderophores in forest soil solution. Biogeochemistry 71(2):247–258
Hou Z, Stack TDP, Sunderland CJ, Raymond KN (1997) Enhanced iron(III) chelation through ligand predisposition: syntheses, structures and stability of tris-catecholate enterobactin analogs. Inorg Chim Acta 263:341–355
Kelly SD, Hesterberg D, Ravel B (2008) Analysis of soils and minerals using X-ray absorption spectroscopy. In: Ulery AL, Drees LR (eds) Methods of soil analysis. Soil Science Society of America, Madison
Khodr HH, Hider RC, Duhme-Klair AK (2002) The iron-binding properties of aminochelin, the mono(catecholamide) siderophore of Azotobacter vinelandii. J Biol Inorg Chem 7(7–8):891–896. doi:10.1007/s00775-002-0375-x
Kohler T, Armbruster T, Libowitzky E (1997) Hydrogen bonding and Jahn–Teller distortion in groutite, α-MnOOH, and manganite, γ-MnOOH, and their relations to the manganese dioxides ramsdellite and pyrolusite. J Solid State Chem 133:486–500
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Kraemer SM, Butler A, Borer P, Cervini-Silva J (2005) Siderophores and the dissolution of iron-bearing minerals in marine systems. In: Banfield JF, Cervini-Silva J, Nealson KH (eds) Molecular geomicrobiology, vol 5. Mineralogical Society of America, Chantilly
Kraepiel AML, Bellenger JP, Wichard T, Morel FMM (2009) Multiple roles of siderophores in free-living nitrogen-fixing bacteria. Biometals 22(4):573–581. doi:10.1007/s10534-009-9222-7
Lee C, Yang W, Parr RG (1988) Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Levina A, Armstrong RS, Lay PA (2005) Three-dimensional structure determination using multiple-scattering analysis of XAFS: applications to metalloproteins and coordination chemistry. Coord Chem Rev 249:141–160
Loomis LD, Raymond KN (1991) Solution equilibria of enterobactin and metal–enterobactin complexes. Inorg Chem 30:906–911
Marschner H, Romheld V, Kissel M (1986) Different strategies in higher plants inmobilization and uptake of iron. J Plant Nutr 9:695–713
Martell AE, Smith RM (2003) Critical stability constant database. National Institute of Science and Technology (NIST), Gaithersburg
Mawji E, Gledhill M, Milton JA, Tarran GA, Ussher S, Thompson A, Wolff GA, Worsfold PJ, Achterbeg EP (2008) Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ Sci Technol 42(23):8675–8680
Mishra B, Haack EA, Maurice PA, Bunker BA (2009) Effects of the microbial siderophore DFO-B on Pb and Cd speciation in aqueous solution. Environ Sci Technol 43(1):94–100
Mucha P, Rekowski P, Kosakowska A, Kupryszewski G (1999) Separation of siderophores by capillary electrophoresis. J Chromatogr A 830(1):183–189
Neu MP, Icopini GA, Boukhalfa H (2005) Plutonium speciation affected by environmental bacteria. Radiochim Acta 93:705
Newville M (2001) IFEFFIT: interactive XAFS analysis and FEFF fitting. J Synchrotron Rad 8:322–324
Parker DL, Sposito G, Tebo BM (2004) Manganese(III) binding to a pyoverdine siderophore produced by a manganese(II)-oxidizing bacterium. Geochim Cosmochim Acta 68:4809–4820
Parker DL, Morita T, Mozafarzadeh ML, Verity R, McCarthy JK, Tebo BM (2007) Inter-relationships of MnO2 precipitation, siderophore–Mn(III) complex formation, siderophore degradation, and iron limitation in Mn(III)-oxidizing bacterial cultures. Geochim Cosmochim Acta 71:5672
Raymond KN, Dertz EA (2004) Biochemical and physical properties of siderophores. In: Crosa JH, Rey AR, Payne SM (eds) Iron transport in bacteria. ASM, Washington, p 3
Rehr JJ, Kas JJ, Prange MP, Sorini AP, Takimoto Y, Vila F (2009) Ab initio theory and calculations of X-ray spectra. C R Phys 10(6):548–559
Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D, Taylor RJ (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123
Roberts JG, Moody BP, McCarty GS, Sombers LA (2010) Specific oxygen-containing functional groups on the carbon surface underlie an enhanced sensitivity to dopamine at electrochemically pretreated carbon fiber microelectrodes. Langmuir 26(11):9116–9122. doi:10.1021/la9048924
Rodgers SJ, Lee CW, Ng CY, Raymond KN (1987) Ferric ion sequestering agents. 15. Synthesis, solution chemistry, and electrochemistry of a new cationic analogue of enterobactin. Inorg Chem 26:1622–1625
Sanford AL, Morton SW, Whitehouse KL, Oara HM, Lugo-Morales LZ, Roberts JG, Sombers LA (2010) Voltammetric detection of hydrogen peroxide at carbon fiber microelectrodes. Anal Chem 82:5205–5210
Schwarzenbach G, Schwarzenbach K (1963) Hydroxamatkomplexe I. Die Stabilität der Eisen(III)-Komplexe einfacher Hydroxamsäuren und des Ferrioxamins B. Helv Chim Acta 46:1390–1400
Schweigert N, Zehnder AJB, Eggen RIL (2001) Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ Microbiol 3(2):81–91
Selig H, Keinath TM, Weber WJ (2003) Sorption and manganese-induced oxidative coupling of hydroxylated aromatic compounds by natural geosorbents. Environ Sci Technol 37:4122–4127
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A32:751–767
Strathmann TJ, Stone AT (2002) Reduction of the pesticides oxamyl and methomyl by Fe(II): effect of pH and inorganic ligands. Environ Sci Technol 36:653–661
Szabo O, Farkas E (2011) Characterization of Mn(II) and Mn(III) binding capability of natural siderophores desferrioxamine B and desferricoprogen as well as model hydroxamic acids. Inorg Chim Acta 376:500–508
Taraz K, Ehlert G, Geisen K, Budzikiewicz H, Korth H, Pulverer Z (1990) Chemicals from bacteria. 40. Protocheline-A catecholate siderophore from a bacterium (DMS no. 5746). Z Naturforsch 45b:1327–1332
Tebo BM, Johnson HA, McCarthy JK, Templeton AS (2005) Geomicrobiology of manganese(II) oxidation. Trends Microbiol 13(9):421–428
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094
Webb SM (2005) SIXPACK: a graphical user interface for XAS analysis using IFEFFIT. Phys Scr T115:1011–1014
Webb SM, Dick GJ, Bargar JR, Tebo BM (2005a) Evidence for the presence of Mn(III) intermediates in the bacterial oxidation of Mn(II). Proc Natl Acad Sci USA 102(15):5558–5563
Webb SM, Tebo BM, Bargar JR (2005b) Structural characterization of biogenic Mn oxides produced in seawater by the marine bacillus sp. strain SG-1. Am Min 90(8–9):1342–1357
Wichard T, Bellenger JP, Loison A, Kraepiel AML (2008) Catechol siderophores control tungsten uptake and toxicity in the nitrogen-fixing bacterium Azotobacter vinelandii. Environ Sci Technol 42(7):2408–2413. doi:10.1021/Es702651f
Winkelmann G (2004) Ecology of Siderophores. In: Crosa JH, Rey AR, Payne SM (eds) Iron transport in bacteria. ASM, Washington, p 437
Winkelmann G (2007) Ecology of siderophores with special reference to the fungi. Biometals 20:379–392
Acknowledgments
We are grateful for support received from the National Science Foundation Geobiology and Low-Temperature Geochemistry Program (award number EAR-0921313). We thank Joe Rogers, Jasquelin Peña, Scott Calvin, and Martin Akafia for help during data collection at the synchrotron. We thank Dorothy Parker for valuable discussion. Portions of this research were carried out at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program (P41RR001209), and the National Institute of General Medical Sciences. Mass spectra were obtained at the NCSU Department of Chemistry Mass Spectrometry Facility by Danielle Lehman. Funding for this facility was obtained from the North Carolina Biotechnology Center, and the NCSU Department of Chemistry.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harrington, J.M., Bargar, J.R., Jarzecki, A.A. et al. Trace metal complexation by the triscatecholate siderophore protochelin: structure and stability. Biometals 25, 393–412 (2012). https://doi.org/10.1007/s10534-011-9513-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9513-7