Abstract
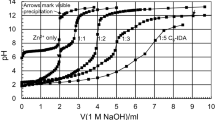
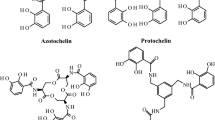
Azotochelin is a biscatecholate siderophore produced by the nitrogen-fixing soil bacterium Azotobacter vinelandii. The complexation properties of azotochelin with a series of oxoanions [Mo(VI), W(VI) and V(V)] and divalent cations [Cu(II), Zn(II), Co(II) and Mn(II)] were investigated by potentiometry, UV–vis and X-ray spectroscopy. Azotochelin forms a strong 1:1 complex with molybdate (log K = 7.6 ± 0.4) and with tungstate and vanadate; the stability of the complexes increases in the order Mo < V < W (log K Moapp = 7.3 ± 0.4; log K Vapp = 8.8 ± 0.4 and log K Wapp = 9.0 ± 0.4 at pH 6.6). The Mo atom in the 1:1 Mo–azotochelin complex is bound to two oxo groups in a cis position and to the two catecholate groups of azotochelin, resulting in a slightly distorted octahedral configuration. Below pH 5, azotochelin appears to form polynuclear complexes with Mo in addition to the 1:1 complex. Azotochelin also forms strong complexes with divalent metals. Of the metals studied, Cu(II) binds most strongly to azotochelin \({(\log \beta_{{{\text{CuLH}}^{{2 -}}}}=-12.9\pm 0.1)}\), followed by Zn(II) \({(\log \beta _{{{\text {ZnL}}^{{3 -}}}} =-24.1\pm 0.14, \log \beta _{{{\text {ZnLH}}^{{2 -}}}} =-17.83\pm 0.09)}\), Mn(II) \({(\log \beta _{{{\text {MnL}}^{{3 -}}}} = -29, \log\beta_{{{\text {MnLH}}^{{2-}}}}=-18.6\pm 0.8, \log \beta _{{{\text {MnLH}}_{2} ^{-}}} =-11.5\pm 0.7)}\) and Co(II) \({(\log \beta _{{{\text {CoLH}}^{{2 -}}}}= -23.0\pm0.3, \log \beta _{{{\text {CoLH}}_{2} ^{-}}}=-13.5\pm 0.2)}\). Since very few organic ligands are known to bind strongly to oxoanions (and particularly molybdate) at circumneutral pH, the unusual properties of azotochelin may be used for the separation and concentration of oxoanions in the laboratory and in the field. In addition, azotochelin may prove useful for the investigation of the biogeochemistry of Mo, W and V in aquatic and terrestrial systems.








Similar content being viewed by others
References
Stiefel EI (2002) In: Sigel A, Sigel H (eds) Molybdenum and tungsten. Their roles in biological processes, vol 39. Dekker, New York, pp 1–30
Kimblin C, Bu XH, Butler A (2002) Inorg Chem 41:161–163
Carter-Franklin JN, Butler A (2004) J Am Chem Soc 126:15060–15066
Bishop PE, Premakumar R, Dean DR, Jacobson MR, Chisnell JR, Rizzo TM, Kopczynski J (1986) Science 232:92–94
Hemrika W, Renirie R, Macedo-Ribeiro S, Messerschmidt A, Wever R (1999) J Biol Chem 274:23820–23827
Messerschmidt A, Wever R (1996) Proc Natl Acad Sci USA 93:392–396
Eady RR (2003) Coord Chem Rev 237:23–30
Kletzin A, Adams MWW (1996) FEMS Microbiol Rev 18:5–63
Chan MK, Mukund S, Kletzin A, Adams MWW, Rees DC (1995) Science 267:1463–1469
Keeler RF, Varner JE (1957) Arch Biochem Biophys 70:585–590
Benemann JR, Smith GM, Kostel PJ, McKenna CE (1973) FEBS Lett 29:219–221
Hales BJ, Case EE (1987) J Biol Chem 262:16205–16211
Siemann S, Schneider K, Oley M, Müller A (2003) Biochemistry 42:3846–3857
Hallenbeck PC, Benemann JR (1980) FEMS Microbiol Lett 9:121–124
Kahn D, Hawkins M, Eady RR (1982) J Gen Microbiol 128:779–787
Lei S, Pulakat L, Gavini N (1999) Biochem Biophys Res Commun 264:186–190
Jacobson MR, Premakumar R, Bishop PE (1986) J Bacteriol 167:480–486
Stintzi A, Barnes C, Xu J, Raymond KN (2000) Proc Natl Acad Sci USA 97:10691–10696
Page WJ, von Tigerstrom M (1982) J Bacteriol 151:237–242
Patel U, Baxi MD, Modi VV (1988) Curr Microbiol 17:179–182
Saxena B, Vithlani L, Modi VV (1989) Curr Microbiol 19:291–295
Duhme AK, Hider RC, Naldrett MJ, Pau RN (1998) J Biol Inorg Chem 3:520–526
Cornish AS, Page WJ (2000) Appl Environ Microbiol 66:1580–1586
Chimiak A, Neilands JB (1984) Struct Bonding 58:89–96
Merz KW, Fink J (1956) Arch Pharm 289:347–358
Tkachev VV, Atovmyan LO (1975) Sov J Coord Chem Engl Transl 1:715–720
Griffith WP, Pumphrey CA, Rainey TA (1986) J Chem Soc Dalton Trans 6:1125–1128
Westall JC, Zachary JL, Morel FMM (1976) Technical Report 18. MIT, Cambridge
Westall JC (1982) Report 82–02. Department of Chemistry, Oregon St University, Corvallis
Herbelin AL, Westall JC (1999) Report 99-01. Department of Chemistry, Oregon St University, Corvallis
Vetrogon VI, Lukyanenko NG, Schwing-Weill MJ, Arnaud-Neu F (1994) Talanta 41:2105–2112
Gans P, Sabatini A, Vacca A (1996) Talanta 43:1739–1753
Martell AE, Smith RM (1974–1989) Critical stability constants. Plenum, New York
Cruywagen JJ (2000) Adv Inorg Chem 49:127–182
Cruywagen JJ, Draaijer AG, Heyns JBB, Rohwer EA (2002) Inorg Chim Acta 331:322–329
Ressler T (1998) J Synchrotron Radiat 5:118–122
Farkas E, Csoka H, Gama S, Santos MA (2000) Talanta 57:935–943
Lu X, Liu S, Mao X, Bu X (2001) J Mol Struct 562:89–94
Atovmyan LO, Sokolova Y, Tkachev VV (1970) Dokl Phys Chem Sect 195:1355–1356
Duhme AK (1997) J Chem Soc Dalton Trans 773–778
Torreggiani A, Trinchero A, Tamba M, Taddei P (2005) J Raman Spectrosc 36:380–388
Cornish AS, Page WJ (1998) Microbiology 144:1747–1754
Boukhalfa H, Crumbliss AL (2002) BioMetals 15:325–339
Hou Z, Raymond KN, O‘Sullivan B, Esker TW, Nishio T (1998) Inorg Chem 37:6630–6637
Carrano CJ, Cooper SR, Raymond KN (1979) J Am Chem Soc 101:599–604
Eady RR, Robson RL, Richardson TH, Miller RW, Hawkins M (1987) Biochem J 244:197–207
Eady RR, Robson RL (1984) Biochem J 224:853–862
Bishop PE, Jarlenski DML, Hetherington DR (1982) J Bacteriol 150:1244–1251
Lei S, Pulakat L, Gavini N (2000) FEBS Lett 482:149–153
Self WT, Grunden AM, Hasona A, Shanmugam KT (2001) Res Microbiol 152:311–321
Anderson MA, Morel FMM (1982) Limnol Oceanogr 27:789–813
Sunda WG, Huntsman SA (1997) Nature 390:389–392
Sunda WG, Huntsman SA (1995) Mar Chem 50:189–206
Sunda WG, Swift DG, Huntsman SA (1991) Nature 351:55–57
Timmermans KR, Stolte W, de Baar HJW (1994) Mar Biol 121:389–396
Wilhelm SW, Trick CG (1994) Limnol Oceanogr 39:1979–1984
Page WJ (1995) BioMetals 8:30–36
Duhme AK, Hider RC, Khodr HH (1997) Chem Ber/Recueil 130:969–973
Knosp O, von Tigerstrom M, Page WJ (1984) J Bacteriol 159:341–347
Acknowledgements
The authors wish to thank Norbert Clauer for his help throughout this work. This study was supported by grants from the NSF (CHE-0221978, Center for Environmental Bioinorganic Chemistry) and the French Department of Research, as well as a fellowship from the French Department of Education to J.P.B.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bellenger, JP., Arnaud-Neu, F., Asfari, Z. et al. Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J Biol Inorg Chem 12, 367–376 (2007). https://doi.org/10.1007/s00775-006-0194-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-006-0194-6