Abstract
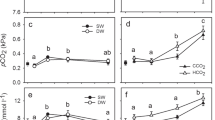
Tidal freshwater ecosystems experience acute seawater intrusion associated with periodic droughts, but are expected to become chronically salinized as sea level rises. Here we report the results from an experimental manipulation in a tidal freshwater Zizaniopsis miliacea marsh on the Altamaha River, GA where diluted seawater was added to replicate marsh plots on either a press (constant) or pulse (2 months per year) basis. We measured changes in porewater chemistry (SO42−, Cl−, organic C, inorganic nitrogen and phosphorus), ecosystem CO2 and CH4 exchange, and microbial extracellular enzyme activity. We found that press (chronic) seawater additions increased porewater chloride and sulfate almost immediately, and ammonium and phosphate after 2–4 months. Chronic increases in salinity also decreased net ecosystem exchange, resulting in reduced CO2 and CH4 emissions from press plots. Our pulse treatment, designed to mimic natural salinity incursion in the Altamaha River (September and October), temporarily increased porewater ammonium concentrations but had few lasting effects on porewater chemistry or ecosystem carbon balance. Our findings suggest that long-term, chronic saltwater intrusion will lead to reduced C fixation and the potential for increased nutrient (N, P) export while acute pulses of saltwater will have temporary effects.





Photo credit: Chris Craft
Similar content being viewed by others
Change history
28 October 2019
In the original publication, in the Methods section at the end of the section Gas Exchange Measurements, the following information was missing: ���For methane, we dropped one to two data points, as appropriate, to improve the r squared of the regression of methane concentration versus time to ���0.85. These changes did not affect the findings of the paper.���
28 October 2019
In the original publication, in the Methods section at the end of the section Gas Exchange Measurements, the following information was missing: ���For methane, we dropped one to two data points, as appropriate, to improve the r squared of the regression of methane concentration versus time to ���0.85. These changes did not affect the findings of the paper.���
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Ardón M, Morse JL, Colman BP, Bernhardt ES (2013) Drought-induced saltwater incursion leads to increased wetland nitrogen export. Glob Change Biol 19:2976–2985
Ardón M, Helton AM, Bernhardt ES (2016) Drought and saltwater incursion synergistically reduce dissolved organic carbon export from coastal freshwater wetlands. Biogeochemistry 127(2–3):411–426
Asmala E, Bowers DG, Autio R, Kaartokallio H, Thomas DN (2014) Qualitative changes of riverine dissolved organic matter at low salinities due to flocculation. Biogeosciences 119:1919–1933
Baldwin AH, Mendelssohn IA (1998) Effects of salinity and water level on coastal marshes: an experimental test of disturbance as a catalyst for vegetation change. Aquat Bot 61:255–268
Barendregt A, Whigham D, Baldwin A (2009) An introduction to the ecosystem. In: Barendregt A, Whigham D, Baldwin A (eds) Tidal freshwater wetlands. Backhuys-Weikersheim, Leiden
Bartlett KB, Bartlett DS, Harriss RC, Sebacher DI (1987) Methane emissions along a salt marsh salinity gradient. Biogeochemistry 4:183–202
Bell CW, Fricks BE, Rocca JD, Steinweg JM, McMahon SK, Wallenstein MD (2013) High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J Vis Exp 81:e50961
Birch JB, Cooley JL (1982) Production and standing crop patterns of giant cutgrass (Zizaniopsis miliacea) in a freshwater tidal marsh. Oecologia 52(2):230–235
Burgin AJ, Hamilton SK (2007) Have we overemphasized denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Capone GD, Kiene RP (1988) Comparison of microbial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33(4, part 2):725–749
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75:2000–2007
Chambers LG, Davis SE, Troxler T, Boyer JN, Downey-Wall A, Scinto LJ, Scinto ADLJ (2013a) Biogeochemical effects of simulated sea level rise on carbon loss in an Everglades mangrove peat soil. Hydrobiologia 726:195–211
Chambers LG, Osborne TZ, Reddy KR (2013b) Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115:363–383
Church JA, Clark PU, Cazenave A, Gregory JM, Jevrejeva S, Levermann A, Merrifield MA, Milne GA, Nerem RS, Nunn PD, Payne AJ, Pfeffer WT, Stammer D, Unnikrishnan AS (2013) Sea Level Change. In: Stocker TD, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1137–1216
Craft C (2007) Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S tidal marshes. Limnol Oceanogr 52:1220–1230
Edmonds JW, Weston NB, Joye SB, Mou X, Moran MA (2009) Microbial community response to seawater amendment in low-salinity tidal sediments. Microb Ecol 58:558–568
Flynn KM, McKee KL, Mendelssohn IA (1995) Recovery of freshwater marsh vegetation after a saltwater intrusion event. Oecologia 103:63–72
Freeman C, Nevison G, Hughes S, Reynolds B, Hudson J (1998) Enzymatic involvement in the biogeochemical responses of a Welsh peatland to a rainfall enhancement manipulation. Biol Fertil Soils 27:173–178
Gardner WS, Seitzinger SP, Malczyk JM (1991) The effects of sea salts on the forms of nitrogen released from estuarine and freshwater sediments: does ion pairing affect ammonium flux? Estuaries 14:157–166
Giblin AE, Weston NB, Banta GT, Tucker J, Hopkinson CS (2010) The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuar Coasts 33:1054–1068
Guo H, Pennings SC (2012) Mechanisms mediating plant distributions across estuarine landscapes in a low-latitude tidal estuary. Ecology 93:90–100
Hackney CT, Avery GB (2015) Tidal wetland community response to varying levels of flooding by saline water. Wetlands 35:227–236
Hart BT, Bailey P, Edwards R, Hortle K, James K, Mcmahon A, Meredith C (1990) Effects of salinity on river, stream and wetland ecosystems in Victoria, Austraila. J Water Res 24:1103–1117
Herbert E, Boon P, Burgin AJ, Neubauer SC, Franklin RB, Ardon M, Hopfensperger KN, Lamers L, Gell P (2015) A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6(10):206
Hopkinson CS (1992) A comparison of ecosystem dynamics in freshwater wetlands. Estuaries 15:549
Horton BP, Rahmstorf S, Engelhart SE, Kemp AC (2014) Expert assessment of sea-level rise by AD 2100 and AD 2300. Quat Sci Rev 84:1–6
Jackson CR, Vallaire SC (2009) Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29:277–287
Johns C, Ramsey M, Bell D, Vaughton G (2014) Does increased salinity reduce functional depth tolerance of four non-halophytic wetland macrophyte species? Aquat Bot 116:13–18
Jun M, Altor AE, Craft CB (2013) Effects of increased salinity and inundation on inorganic nitrogen exchange and phosphorus sorption by tidal freshwater floodplain forest soils, Georgia (USA). Estuar Coasts 36:508–518
Kang H, Freeman C (1999) Phosphatase and arylsulphatase activities in wetland soils: annual variation and controlling factors. Soil Biol Biochem 31:449–454
Kang H, Kim SY, Fenner N, Freeman C (2005) Shifts of soil enzyme activities in wetlands exposed to elevated CO2. Sci Total Environ 337:207–212
Ket WA, Schubauer-Berigan JP, Craft CB (2011) Effects of five years of nitrogen and phosphorus additions on a Zizaniopsis miliacea tidal freshwater marsh. Aquat Bot 95:17–23
Kong L, Wang YB, Zhao LN, Chen ZH (2009) Enzyme and root activities in surface-flow constructed wetlands. Chemosphere 76:601–608
Lamers LPM, Els Ten Dolle G, Van Den Berg STG, Van Delft SPJ, Roelofs JGM (2001) Differential responses of freshwater wetland soils to sulphate pollution. Biogeochemistry 55:87–102
Lamers LPM, Govers LL, Janssen ICJM, Geurts JJM, Van der Welle MEW, Van Katwijk MM, Van der Heide T, Roelofs JGM, Smolders AJP (2013) Sulfide as a soil phytotoxin-a review. Front Plant Sci 4:268
Lamers LPM, Falla S, Samborska EM, Van Dulken IAR, Hengstum V, Roelofs JGM (2014) Factors controlling the extent of eutrophication and toxicity in sulfate-polluted freshwater wetlands. Limnol Oceanogr 47:585–593
Lovley DR, Klug MJ (1986) Model for the distribution of sulfate reduction and methanogenesis in freshwater sediments. Geochim Cosmochim Acta 50:11–18
Madrid EN, Armitage AR, Quigg A (2012) The response of photosystem II to soil salinity and nutrients in wetland plant species of the northwestern Gulf of Mexico. J Coast Res 284:1197–1207
Marton JM, Herbert ER, Craft CB (2012) Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands 32:347–357
Montagna PA, Alber M, Doering P, Connor MS (2002) Freshwater inflow: science, policy, management. Estuaries 25:1243–1245
Morris JT, Sundareshwar PV, Nietch CT, Kjerve B, Cahoon DR (2002) Responses of coastal wetlands to rising sea level. Ecology 83:2869–2877
Morrissey EM, Berrier DJ, Neubauer SC, Franklin RB (2013) Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry 117:473–490
Morrissey EM, Gillespie JL, Morina JC, Franklin RB (2014) Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob Change Biol 20:1351–1362
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nelson TM, Streten C, Gibb KS, Chariton AA (2015) Saltwater intrusion history shapes the response of bacterial communities upon rehydration. Sci Total Environ 502:143–148
Neubauer SC (2008) Contributions of mineral and organic components to tidal freshwater marsh accretion. Estuarine 78:78–88
Neubauer SC (2013) Ecosystem responses of a tidal freshwater marsh experiencing saltwater intrusion and altered hydrology. Estuar Coasts 36:491–507
Neubauer SC, Craft CB (2009) Global change and tidal freshwater wetlands: scenarios and impacts. In: Barendregt A, Whigham D, Baldwin A (eds) Tidal freshwater wetlands. Backhuys-Weikersheim, Leiden, pp 253–266
Neubauer SC, Megonigal JP (2015) Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 18(6):1000–1013
Neubauer SC, Miller DW, Anderson IC (2000) Carbon cycling in a tidal freshwater marsh ecosystem: a carbon gas flux study. Mar Freshw Res 199:13–30
Neubauer SC, Givler K, Valentine S, Megonigal JP (2005) Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86:3334–3344
Neubauer SC, Franklin RB, Berrier DJ (2013) Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10:10685–10720
Nielsen DL, Brock MA, Rees GN, Baldwin DS (2003) Effects of increasing salinity on freshwater ecosystems in Australia. Aust J Bot 51:655
Nijssen B, O’Donnell GM, Hamlet AF, Lettenmaier DP (2001) Hydrologic sensitivity of global rivers to climate change. Clim Change 50:143–175
Noe GB, Krauss KW, Lockaby BG, Conner WH, Hupp CR (2013) The effect of increasing salinity and forest mortality on soil nitrogen and phosphorus mineralization in tidal freshwater forested wetlands. Biogeochemistry 114:225–244
Odum W (1988) Comparative ecology of tidal freshwater and salt marshes. Annu Rev Ecol Syst 19:147–176
Paludan C, Morris JT (1999) Distribution and speciation of phosphorus along a salinity gradient in intertidal marsh sediments. Biogeochemistry 45:197–221
Parris A, Bromirski P, Burkett V, Cayan D, Culver M, Hall J, Horton R, Knuuti K, Moss R, Obeysekera J, Sallenger A, Weiss J (2012) Global Sea Level Rise Scenarios for the United States National Climate Assessment. NOAA Technical Report OAR CPO-1. Climate Program Office, Silver Springs, MD, USA
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–842
Prat N, Ibanez C (1995) Effects of water transfers projected in the Spanish National Hydrological Plan on the ecology of the lower river Ebro (N.E. Spain) and its delta. Water Sci Technol 31:79–86
Rickard D, Morse JW (2005) Acid volatile sulfide (AVS). Mar Chem 97:141–197
Rysgaard S, Thastum P, Dalsgaard T, Christensen PB, Sloth NP, Rysgaard S (1999) Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22:21
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Segarra KEA, Comerford C, Slaughter J, Joye SB (2013) Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim Cosmochim Acta 115:15–30
Shackle VJ, Freeman C, Reynolds B (2000) Carbon supply and the regulation of enzyme activity in constructed wetlands. Soil Biol Biochem 32:1935–1940
Sharpe PJ, Baldwin AH (2012) Tidal marsh plant community response to sea-level rise: a mesocosm study. Aquat Bot 101:34–40
Sinsabaugh RL, Findlay S (1995) Microbial production, enzyme activity, and carbon turnover in surface sediments of the Hudson River estuary. Microb Ecol 30:127–141
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. https://websoilsurvey.sc.egov.usda.gov/. Accessed 4 July 2015
Spalding E, Hester M (2007) Interactive effects of hydrology and salinity on oligohaline plant species productivity: implications of relative sea-level rise. Estuar Coasts 30:214–225
Sutter LA, Perry JE, Chambers RM (2013) Tidal freshwater marsh plant responses to low level salinity increases. Wetlands 34:167–175
Sutton-Grier AE, Megonigal JP (2011) Plant species traits regulate methane production in freshwater wetland soils. Soil Biol Biochem 43:413–420
Tobias C, Neubauer SC (2009) Saltmarsh biogeochemistry: an overview. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands: an integrated ecosystem approach. Elsevier, Amsterdam, pp 445–492
Van Der Nat FJWA, Middelburg JJ (1998) Effects of two common macrophytes on methane dynamics in freshwater sediments. Biogeochemistry 43:79–104
Van Diggelen JMH, Lamers LPM, van Dijk G, Schaafsma MJ, Roelofs JGM, Smolders AJP (2014) New insights into phosphorus mobilisation from sulphur-rich sediments: time-dependent effects of salinisation. PLoS ONE 9:e111106
Weston NB, Dixon RE, Joye SB (2006) Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. J Geophys Res 111:G01009
Weston NB, Giblin AE, Banta GT, Hopkinson CS, Tucker J (2010) The effects of varying salinity on ammonium exchange in estuarine sediments of the Parker River, Massachusetts. Estuar Coasts 33:985–1003
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102:135–151
Whiting GJ, Bartlett DS, Fan SM, Bakwin PS, Wolfsy SC (1992) Biosphere atmosphere CO2 exchange in tundra ecosystems – community characteristics and relationships with multispectral surface reflectance. J Geophys Res 97(D15):16671–16680
Williams WD (1999) Salinisation: a major threat to water resources in the arid and semi-arid regions of the world. Lakes Reserv 4:85–91
Williams AA, Lauer NT, Hackney CT (2014) Soil phosphorus dynamics and saltwater intrusion in a florida estuary. Wetlands 34:535–544
Acknowledgements
We thank the students and technicians who participated in the SALTEx project, especially Dontrece Smith, M. Maurer, C. Peacock, and the many IU Wetlands Laboratory students and GCE LTER Schoolyard participants who helped build the porewater wells and measure porewater and gas fluxes. A special thanks to Sarah Widney and two anonymous reviewers who made substantive improvements to the manuscript. This research was supported by funding from the National Science Foundation to CBC through the NSF LTER program (Georgia Coastal Ecosystems LTER, OCE-9982133, OCE-0620959 and OCE-1237140) and to ERH through the NSF GRFP (2011117001) and NSF DEB DDIG program (DEB-1401070) and support from the U.S. EPA ORD to JSB. This is contribution 1065 of the University of Georgia Marine Institute. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the USEPA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: R. Kelman Wieder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herbert, E.R., Schubauer-Berigan, J. & Craft, C.B. Differential effects of chronic and acute simulated seawater intrusion on tidal freshwater marsh carbon cycling. Biogeochemistry 138, 137–154 (2018). https://doi.org/10.1007/s10533-018-0436-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0436-z