Abstract
We used established long-term experimental P-amended plots in freshwater marshes of northern Belize to determine the impact of P input on nitrogen (N) fixation. Marshes with different conductivities and sulfate concentrations were selected to elucidate the effect of salinity and the contribution of sulfur reducing bacteria to the overall N fixation. Rates of N fixation in sediment, roots, and cyanobacterial mats was measured in laboratory incubation experiments (acetylene reduction assay calibrated by 15N2 reduction assay) with and without the addition of sodium molybdate (sulfur reducing bacteria inhibitor). P has increased macrophyte primary production significantly, which led to the rapid elimination of cyanobacterial mats and the elimination of autotrophic N fixation. P addition enhanced heterotrophic N fixation in both the sediments and rhizosphere due primarily to increased C supply to the sediment. When expressed on a dry weight basis, root associated N fixation was higher than sediment N fixation, but the contribution of the root associated fixation to the total N fixation was small when expressed per square meter. Sulfur reducing bacteria were an important component of N fixation, contributing from 20 to 53% to the overall N fixation. A simple N budget was created to determine if N demands are met following P addition. The heterotrophic N fixation substituted in part for autotrophic cyanobacterial N fixation when P limitation was alleviated.
Similar content being viewed by others
Introduction
Research on biological nitrogen (N) fixation and its role in maintaining soil N fertility in wetland ecosystems has focused on coastal wetlands such as salt marshes, mangroves (Pelegri and Twilley 1998; Bagwell and Lovell 2000; Nielsen et al. 2001; Tyler et al. 2003; Lee and Joye 2006; Moseman 2007), and on rice fields (Eskew et al. 1981; Roger and Ladha 1992; Roger 1995). Data from freshwater wetlands, however, are rather limited (Bristow 1973; Tjepkema and Evans 1976; Scott et al. 2008; Černá et al. 2009).
Two types of organisms are often responsible for N fixation in wetlands: autotrophic cyanobacteria, which occur in benthic, floating, or periphytic cyanobacterial mats (Rejmánková and Komárková 2000; Rejmánková et al. 2004; Inglett et al. 2004, 2009; Scott et al. 2005, 2007; Lee and Joye 2006), and heterotophic N fixers in the rhizosphere or in bulk sediments (Moseman 2007; Černá et al. 2009). Autotrophic N fixation prevails in oligotrophic and mesotrophic wetlands where cyanobacterial mats are often the main primary producers because macrophytes are nutrient limited (Rejmánková and Komárková 2000; Scott et al. 2007). After eutrophication, however, the growth of macrophytes often increases, replacing cyanobacteria, and thus reducing autotrophic N fixation. We have reported a significant increase in heterotrophic N fixation in sediments exposed to nutrient, namely phosphorus, enrichment (Černá et al. 2009). It is plausible that the increased activity of heterotrophic bacteria might help to replace the input of N that was originally derived from cyanobacterial N fixation.
Since the triple bond of atmospheric N2 is stable, the reduction of N2 to ammonia during its biological fixation is an expensive process energetically. Therefore diazotroph incidence and activity depends on the availability of energy rich carbon (C) source. The continual input of easily available C into the plant rhizosphere sustains high activity of root associated microflora (Boyle and Patriquin 1981; Bergholz et al. 2001; Nielsen et al. 2001; Moseman 2007), and thus diazotrophs are closely associated with plant roots (Dakora and Drake 2000; Bürgmann et al. 2005). In addition to C availability, the activity of diazotrophs in wetland sediments is repressed by high concentrations of ammonium (Howarth et al. 1988; Černá et al. 2009) and low concentrations of available phosphorus (P) (Reed et al. 2007).
Diazotrophs comprise a taxonomically diverse group that includes aerobes, microaerophiles, facultative and strict anaerobes (Capone and Kiene 1988). Anaerobes of particular importance in wetland and water ecosystems are represented by Enterobacter spp., Klebsiella spp., Vibrio spp., Desulfobacter spp., Desulfovibrio spp. and Clostridium spp. (Herbert 1999).
Sulfate-reducing bacteria can fix N and diazotrophy is widespead among mezophilic sulfur reducing bacteria (Riederer-Henderson and Wilson 1970; Postgate and Kent 1985; Welsh et al. 1996b; Steppe and Paerl 2002). The abundance and activity of sulfur reducing bacteria is driven by anoxia, the presence of sulfates and high C availability. Reports of the important contributions of sulfur reducing bacteria to N fixation have been limited to coastal marine sediments (Capone 1982; Capone and Kiene 1988; Welsh et al. 1996b; Kusel et al. 1999). However, if the above conditions are met, diazotrophic activity of sulfur reducing bacteria can be expected in any wetland ecosystem, including inland freshwater wetlands.
N fixation is assessed using acetylene reduction assay (ARA) with a substrate analog, acetylene (C2H2). The rate of C2H2 reduction is converted to the rate of N fixation using an estimate of the C2H2:N2 reduction ratio. The theoretical reduction ratio is 3:1, reflecting the number of reducing equivalents required by nitrogenase to reduce C2H2 to C2H4 relative to the number required to reduce N2 to NH4 (Zehr and Montoya 2007). However, this ratio can vary from 1.5:1 to 100:1 (summarized in Howarth et al. 1988) and a direct calibration through 15N fixation assay is needed to get reliable estimates of N2 input to the ecosystem.
Here we report the heterotrophic N fixation and contribution of sulfur reducing bacteria to N fixation in sediment and rhizosphere of Typha domingensis and Eleocharis spp. from inland wetlands in northern Belize. These wetlands are limestone based marshes that vary in size from small <1 ha marshes to large shallow lagoons with water conductivities ranging from 0.2 to 7 miliSiemens. The marshes are strongly limited by P, although the status of some of them has been changing recently due to increasing input of nutrients from fertilizer runoff from sugar cane fields and other crops (Johnson and Rejmánková 2005). The unimpacted marshes are dominated by benthic cyanobacterial mats with scattered macrophytes, mainly Eleocharis spp. (Rejmánková and Komárková 2005). In 2001, we initiated a long term manipulative experiment using these wetlands as a model system, to obtain a mechanistic explanation for an ecosystem level response to increased nutrient input across a salinity gradient. We have already confirmed that P addition leads to almost total elimination of cyanobacterial mats due to the expansion of Eleocharis cellulosa, and, eventually, the replacement of Eleocharis by Typha domingensis (Rejmánková et al. 2008). We hypothesized that the loss of the N input from the autotrophic N fixation by cyanobacterial mats can be replaced by the increased heterotrophic N fixation in the rhizosphere and sediments (Fig. 1). The activities of heterotrophic diazotrophs in sediment increased with higher input of organic C and a more favorable C/P ratio of the plant litter (Černá et al. 2009).
Simplified diagram of organisms, nutrient pools and interaction pathways in the study system (interactions are not all inclusive to facilitate the clarity of the diagram). The pathways that are quantified are indicated in gray; thickness of the arrows indicates a rate of C input and N fixation, respectively
We focus on the following hypotheses:
-
(1)
Total N fixation will be higher in rhizosphere than in sediment and will be enhanced by P addition, due to the higher input of available C in rhizosphere.
-
(2)
Sulfur reducing bacteria will contribute to the overall diazotrophic activity as the studied marshes have conditions suitable for sulfur reducing bacteria development, with fair concentrations of sulfate in sediment and interstitial water.
-
(3)
The input of N2 from autotrophic N fixation can be replaced by heterotrophic N fixation after P addition. In our preliminary study, we found P enriched soils to have dramatically higher heterotrophic fixation rates than control soils (Černá et al. 2009).
To test these hypotheses, we measured diazotrophic activities of sediments, roots, and litter in laboratory incubation experiments with and without the addition of sodium molybdate, a ‘specific’ metabolic inhibitor for sulfur reducing bacteria (Oremland and Capone 1988). Nitrogenase activity was measured using the acetylene reduction assay, which was calibrated by 15N2 reduction assay. Using these values and other data from the long term experiment, we created a simple N budget to determine if N demands are met following P addition.
Material and methods
Study site
Our study sites are located in lowlands of northern Belize, Central America, in the Orange Walk and Corozal districts. A detailed description has been provided elsewhere (Černá et al. 2009; Rejmánková et al. 2008). Briefly: the limestone geology and occasional intrusion of seawater result in a diverse range of water conductivities with large differences in sulfate, bicarbonate and chloride. The climate of the region is tropical wet–dry. The majority of wetlands in the study area remain flooded or water saturated year round, although the total flooded area may vary as water levels rise and fall.
Main primary producers in these systems are several species of emergent macrophytes (Eleocharis cellulosa, E. interstincta, Cladium jamaicense and Typha domingensis) and species rich communities of microphytes represented mostly by cyanobacteria (Rejmánková et al. 2004). Both macro- and microphytes in these wetlands are P limited as has been confirmed in cyanobacterial mats and Eleocharis dominated marshes (Rejmánková and Komárková 2000; Rejmánková 2001). No nitrogen limitation has been detected in any of the reported studies.
Fifteen marshes, five in each low, medium and high salinity category, dominated by sparse macrophytes (Eleocharis spp.) were studied as a part of a project to assess ecosystem response to nutrient addition along a salinity gradient. Four 10 × 10 m plots were established in each marsh in August of 2001, one represents a control, the remaining three received N, P and N&P addition in August 2001, August 2002 and March 2005. N was added as ammonium nitrate and P as triple super phosphate in amounts corresponding to 20 and 10 g m−2 y−1, respectively. In March 2003, one individual of Typha domingensis was planted in each plot. In the majority of controls and N addition plots Typha plants have not survived, while in P-amended plots they have been growing vigorously. In January of 2005, P-amended plots in six marshes were manipulated to be half dominated by Eleocharis and half dominated by Typha. Samples for this study were selected from four of these six marshes (Table 1), two of low (LS) and two of high salinity (HS).
Sediment and root sampling and analyses
In August 2007 (beginning of the wet season) and March 2009 (middle of the dry season), sediment samples were collected from control (LP) and P-amended (HP) Eleocharis dominated (E) and Typha dominated (T) plots. The plots were always flooded by 20 or more cm of water during the time of sampling. Recently deposited, readily distinguishable plant litter on the soil surface was removed gently before sampling. From each sampling site, three randomly located sediment samples were collected to a depth of approximately 10 cm and sealed in a plastic bag. Typha and Eleocharis root samples were collected from the same plots as sediment samples in August 2007 (beginning of wet season), March 2008 and 2009 (middle of dry seasons). Four plants were extracted carefully out of sediment to prevent extensive root damage and roots, washed in surface water to remove adhered particles and sealed in a plastic bag. Four samples of floating cyanobacterial mats, approximately 10 × 10 cm in size, were collected from control plots in 2002, 2004, 2008 and 2009 and placed in shallow containers with a small amount of marsh water. From each plot, interstitial water was collected and transferred into 3 l plastic bottle. All of the above samples were transported in a cooling box to the laboratory and transferred immediately to a refrigerator. Samples were processed within 8 h after sampling, as the activity of sulfate reducers was not affected after exposure to air for 8 h (Blaabjerg and Finster 1998).
Acetylene reduction assay (ARA)
Live roots were identified by color and structure, and an equivalent of ~200 mg DW were transferred to 100 ml containers with 50 ml of interstitial water from the corresponding site. Bulk sediment was obtained by removing plant material from sediment with pair of forceps and 30 g were transferred to 100 ml containers with 20 ml interstitial water from the corresponding site. Just prior to incubation, glucose (final concentration 2 mmol l−1) was added to each sample. The activity of sulfur reducing bacteria on roots and in sediment was eliminated by the addition of sodium molybdate (NaMoO4, final concentration 20 mmol l−1) together with glucose (Nielsen et al. 2001). Nitrogenase activity of sulfur reducing bacteria was calculated as the difference between untreated and treated with NaMoO4 samples. The bottles were equilibrated to atmospheric pressure, and 30 ml of headspace were removed, which was replaced with 20 ml of N2 gas to lower the partial pressure of oxygen. Ten milliliters of C2H2, prepared freshly from CaC2, were added to each bottle, which were then shaken vigorously and incubated under dark conditions at 28–30°C for 24 h. At the end of the incubation, several ml of headspace were withdrawn with an airtight syringe and analyzed by a gas chromatograph, Shimadzu 14 GC with a flame ionization detector and a Porapak-T column at 80°C. Controls run with samples without acetylene addition as well as blanks (empty bottles incubated with 10 ml of acetylene) showed no endogenous ethylene production.
For cyanobacterial mats fixation, we cut three 3 cm diameter circles from each collected cyanobacterial mats, using a cookie-cutter. Each circle was placed in a bottle with 50 ml of the respective marsh water and incubated without glucose addition. To determine any differences between day and night cyanobacterial mats fixation, the cyanobacterial mats samples were incubated outdoors for 4 h during the day (11 am–3 pm), while an independent set of samples were incubated for 8 h during the night (10 pm–6 am).
In 2007, 2008 and 2009, the dependence of the overall nitrogenase activity on C substrate (glucose) availability was tested. Nitrogenase activity was measured on composite sediment samples after the addition of glucose to final concentrations of 0, 0.016, 0.08, 0.16 and 0.32 mg C ml−1.
In 2007, the effect of sulfate concentration on nitrogenase activity of sulfur reducing bacteria was determined. Nitrogenase activity was measured on composite Typha and Eleocharis root samples placed in interstitial water from high salinity HP plots with the addition of K2SO4 to the concentration typical for sea water (2.45 g l−1).
15N reduction assay
The measurements were done as for the ARA but 10 ml of 15N2 (99atom%, Cambridge Isotope) was added instead of C2H2. At the end of the incubation, the content of containers was frozen, freeze dried and ground on a Wiley mill. The initial 15N enrichment of the sample was determined from the ARA samples. The δ15N (in relation to atmospheric N2 as the reference standard material) was measured by an isotope ratio mass spectrometer (IR-MS Delta X Plus, Finnigan, Germany). Biomass specific N fixation rate, normalized to organic N, was calculated as isotopic balance (Montoya et al. 1996; Zehr and Montoya 2007):
Where PN is N concentration in the sample, AP is 15N enrichment (atom% 15N) of the sample or substrate (N2) pool at the beginning (initial) and end (final) of incubation; Δt is the length of incubation. N fixation rate expressed in terms of fixation of molecular N2 to organic material was then calculated:
Phopsholipid fatty acid (PLFA) analysis
PLFA analyses were performed in sediment (~2 g fresh weight) sampled in 2007 according to Frostegård et al. (1993). Phospholipids, in form of fatty acid methyl esters (FAME) were quantified by gas chromatography Hewlett Packard 6890 with FID detector. Since the quantity of microbial PLFA is a function of microbial biomass (Zelles et al. 1992), we used the total amount of all bacterial PLFA identified in the samples as an index of live microbial biomass. Terminally branched monounsaturated PLFA i17:1, a17:1, i18:1, typical for Desulfovibrio spp. (Kohring et al. 1994) and 10Me16:0 typical for Desulfobacter spp. (Taylor and Parkes 1983; Londry et al. 2004) were used as sulfur reducing bacteria biomarkers.
Estimation of N input via N fixation and budget construction
The nitrogenase activity measured using ARA was converted to the actual N fixation activity using the experimentally estimated reduction ratio of C2H4 to N2.
The N budget was constructed as the balance between macrophyte N needs and N inputs through N fixation and precipitation. Macrophyte N needs were calculated as the difference between the proportion of total N in biomass minus amount of N that is resorbed from the senescing tissues.
where NPP is the biomass produced per year (g m−2) and Ntissue is the amount of N in the biomass (%).
where NRE is the nitrogen resorption efficiency (%), i.e., the proportion of N that is resorbed from the senescing tissues (for details on net primary production and N resorption efficiency see Rejmánková and Snyder (2008). For N input other than from fixation, we included N from precipitation. N from precipitation was estimated to be 0.36 g m−2 y−1 of combined input of NH4–N and NO3–N, based on McDowell (1998) and Borbor-Cordova et al. (2006). None of the study sites has any significant surface inflow and the ground water discharge is probably a minor component of the water budget, considering that the average precipitation is 1400 mm and potential evapotranspiration is 1490 mm (King et al. 1992). For contribution of N fixation by cyanobacteria, we used the average values of N fixation measurements (also see Rejmánková and Komárková 2005; Rejmánková unpublished). The amount of fixed N per square meter per year was estimated from the average cyanobacterial biomass data from the 2001 marsh surveys that resulted in values of 1026 and 925 g m−2 for low and high salinity marshes, respectively (Rejmánková, unpublished). These values were in a close agreement with the primary production data of 1024 and 691 g m−2 y−1 measured by Rejmánková and Komárková (2005).
Two scenarios: (A) Potential and (B) Estimated actual heterotrophic N fixation provided estimates of heterotrophic N fixation. Potential heterotrophic N fixation under conditions of unlimited C supply was estimated from our measurements. The measured values of N fixation were adjusted to the actual C input from root exudates and decomposition for heterotrophic rhizosphere and sediment N fixation, respectively, to estimate actual heterotrophic N fixation. The amount of C exuded by roots was estimated as 20% of total NPP using the 13C labeling approach (Šantrůčková et al. 2009) assuming that all exuded C was used by root associated microflora. The amount of C from decomposition was estimated as C released in the process of decomposition of shoots and roots (for decomposition rates see Rejmánková and Houdková 2006; Snyder et al. in preparation).
The N fixation activity of the sediment was calculated for the upper 5 cm sediment layer and after adjusting for sediment bulk density, was expressed in g N m−2y−1. To express the rhizosphere N fixation, we used data on belowground root NPP, which was calculated from each plot’s quarterly collected sediment in-growth cores (n = 3) (Snyder et al, in preparation). Briefly, each in-growth core was constructed of 2 × 3 mm flexible mesh, filled with of native marsh soil devoid of roots, and incubated in the soil of each plot, allowing roots to grow within it for 3 months. Upon careful the excavation of each in-growth core, the root biomass was removed and separated into live and dead roots (for methodology see Černá et al. 2009)
Data analysis
Nitrogenate activity was measured in three (sediment) or four (roots) replicates, each from one independent sample randomly collected from each treatment. We used repeated measures ANOVA with a year as within-subject factor with Neumann-Keuls Post Hoc test (STATISTICA version 8.0) to evaluate: (1) the effect of P addition, salinity, and sodium molybdate on sediment nitrogenase activity, comparing LP/E and HP/E plots at the high and low salinity sites; (2) the effect of plant species, salinity, and sodium molybdate on sediment nitrogenase activity, comparing HP/E and HP/T at the high and low salinity sites and (3) the effect of plant, P addition, salinity, and sodium molybdate on root nitrogenase activity, comparing all the plots. We could not compare all the plots evaluating sediment nitrogenase activity due to the unbalanced design (there are no samples of low P and Typha). Factorial ANOVA was used to evaluate the effect of sulfate addition. Linear correlation was used to estimate the relationship between sediment and rhizosphere nitrogenase activity, while linear regression estimated nitrogenase activity dependency on C availability. To estimate the uncertainty of the computed N budgets, an error propagation technique was employed, using the standard errors of all measurements used to construct the budget (Bevington and Robinson 2002).
Results
Calibration of ARA through 15N2 reduction assay
In all types of samples (sediment, roots, cyanobacterial mats), the nitrogenase activity measured by ARA was correlated closely to the amount of organic 15N measured in organic material (r = 0.807, N = 60, y = 2.41x), denoting that the general N2 reduction:C2H4 reduction ratio was 1:2.41. Evaluating the correlation for each type of samples revealed that each had a distinct N2 reduction:C2H4 reduction ratio (Fig. 2). The N2:C2H4 ranged from 0.60 (sediment) to 4.31 (cyanobacterial mats).
N fixation of sediment, roots, litter and cyanobacterial mats
N fixation of all diazotrophs in sediments from control plots was low, with a mean of only 0.229 nmol N g −1dw h−1, and was more than an order of magnitude lower than P-addition plots (Table 2). The increase between control and P addition was lower at high salinity plots, but the effect of salinity was insignificant. In P-amended plots, sediment N fixation was always significantly higher in Typha as compared to Eleocharis plots, and these differences were strongest in the low salinity sites.
When expressed on a g DW basis, the N fixation of root associated diazotrophs always significantly exceeded that of sediment bacteria (Tables 2 and 3). Within control plots of low salinity marshes, N fixation of root associated diazotrophs was higher in Typha than in Eleocharis, but the opposite was true for the high salinity marshes. N fixation of root associated diazotrophs was also affected positively by P addition, but again these differences were strongest in the low salinity sites. In all P-amended plots, N fixation of root associated diazotrophs was higher in Typha than in Eleocharis. Rhizosphere N fixation closely correlated to sediment N fixation (Fig. 3a). Close positive correlation between root biomass and N fixation in the sediment (Fig. 4a) and an exponential increase of C availability in the sediment with an increase of root biomass (Fig. 4b) was found.
The cyanobacterial N fixation varied between sampling dates and marshes (Table 4), but when the amount of N fixed by cyanobacterial mats per year was averaged over several sampling periods, there were no significant differences between the low and high salinity marshes with mean values of 2.20 and 2.99 g N m−2 y−1, respectively.
Nitrogenase activity associated with the sediment and plant litter displayed very similar linear dependency on C availability (glucose addition) in all 3 years tested. The slope of the regression line was 330.5 ± 30.7 (R 2 = 0.97, N = 3).
Contribution of sulfur reducing bacteria to total N fixation
The addition of sodium molydbate reduced N fixation of sediment and root associated bacteria significantly, indicating that a portion of N fixation is contributed by sulfur reducing bacteria (Tables 2 and 3). When evaluating each treatment separately, however, this decrease was significant only in the P-amended plots, apparently due to the high variability of the data from year to year and among replicates.
The contribution of sulfur reducing bacteria-N fixation to the overall N fixation was on average 24 ± 17% and 57 ± 25%, for root associated and sediment bacteria, respectively. In the sediment, the elimination of sulfur reducing bacteria-N fixation caused a significant decrease in N fixation in low salinity P-amended plots (Table 2). Elimination of the sulfur reducing bacteria-N fixation of root associated bacteria also significantly decreased only in the P-amended plots. The decrease was neither salinity nor plant dependent (Table 3). Sulfur reducing bacteria eliminated N fixation of root associated bacteria was correlated closely to that of sediment (Fig. 3b) with the regression line having a lower slope than that for the overall N fixation.
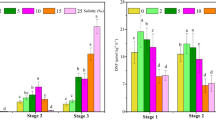
The overall N fixation of Eleocharis and Typha root associated bacteria was not affected significantly by sulfate addition to the interstitial water (Fig. 5). However, the addition of sulfate affected the contributions from the different microflora groups, especially in Typha. The proportion of N fixation from sulfur reducing bacteria increased by 15 and 45% in the rhizospheres of Eleocharis and Typha, respectively. This test was run only with root material from the high salinity plots as we did not want to expose root microflora adjusted to low salinity, to the osmotic shock associated with high sulfate addition.
Total N fixation of root associated microflora from Eleocharis (E) and Typha (T) high salinity P addition plots. The roots were incubated in interstitial water with natural salinity (0) or in interstitial water with K2SO4 (2.5 g l−1). The white portion of the columns refers to sulfur reducing bacteria–N fixation (SRB), while the grey portion refers to non sulfur reducing bacteria–N fixation (non SRB). Vertical bars denote 0.95 confidence limits and asterisks indicate a significant effect of K2SO4 addition (factorial ANOVA, N = 4, P > 0.001)
PLFA biomarkers for Desulfovibrio and Desulfobacter were always detected in the sediment and they represented a relatively constant part of the microbial community (see the comparison between years, Table 5). No significant effect of salinity and phosphorus addition was found.
Estimation of N input via N fixation and budget construction
Estimation of (A) potential heterotrophic N fixation under conditions of unlimited C supply and (B) actual heterotrophic N fixation adjusted for actual C input from root exudates shows that for scenario (A), the heterotrophic N fixation would provide enough new N to cover potential macrophyte N requirements in the P-amended plots (Tables 6 and 7). Under scenario (B), N provided by heterotrophic N fixation in the Eleocharis and Typha dominated P-amended plots would be 72 to 81% and 41 to 82%, respectively, short of plant needs (Table 7). Despite the high N fixation of root associated bacteria expressed on dry weight basis, their contribution to total heterotrophic N fixation was several orders of magnitude lower than the contribution of sediment bacteria, which is due to the lower root than sediment weight per square meter. At control plots, heterotrophic N fixation was negligible and most of the N required by macrophytes was provided by cyanobacterial mats fixation (Tables 6 and 7).
Discussion
N fixation by sediment and root associated diazotrophs
P addition had a significant positive effect on N fixation of both sediment and root associated diazotrophs. This result agrees with our previous data, which illustrated that sediment P affected N fixation positively (Černá et al. 2009). The removal of P limitation in P-amended plots stimulated macrophyte biomass production, which we assumed to provide higher C input into the sediment. Accordingly, we found an exponential increase of C availability in the sediment with an increase of root biomass and a close positive correlation between root biomass and N fixation in the sediment. Root associated N fixation measured in this study was higher than that of sediment N fixation, when expressed on dry weight basis.
The higher N fixation in the rhizosphere can be explained by the higher C availability in the rhizosphere as compared to the sediment. All our results indicate that once P limitation is removed, C primarily limits diazotroph activity.
Root associated rates of N fixation, which we expressed per square meter of soil, was much lower than rates of sediment N fixation. This was caused by the small proportion of root weight in the total sediment weight. It clearly demonstrates that the contribution of root associated N fixation to the total ecosystem N fixation can be overestimated if N fixation is solely expressed on root dry weight.
Role of sulfate reducing diazotrophs
In the majority of cases, sulfur reducing bacteria contributed about 50 and 20% to the overall diazotrophic activity in sediment and rhizosphere samples, respectively. Our hypothesis that this contribution would be higher in marshes with higher sulfate content was not confirmed because there was no difference in the contribution of sulfur reducing bacteria between low and high salinity sites. Although our research plots are all inland wetlands, the limestone geology, at places enriched with gypsum, and the occasional intrusion of seawater result in two orders of magnitude differences in the sulfate content of the interstitial water (Table 1). The sulfate content in the low salinity marshes (1.2–5.8 mg l−1) is well in the range of values for freshwater wetlands (Dodla et al. 2009), while sulfate concentrations of 600–1200 mg l−1 in the high salinity marshes approach about one half of the typical saltmarsh sulfate concentrations (Koretsky et al. 2005). As far as we know, N fixation activity of sulfur reducing bacteria has not been assumed to be important in freshwater wetlands, but the presented results document that sulfur reducing diazotrophs play an important role even in low salinity marshes. Their contribution is substantially less than in marine systems, where sulfur reducing bacteria are the dominant N fixers, contributing 75–90% of overall N fixation (Welsh et al. 1996a), yet they are a significant component of N fixation. We believe that if the marshes in our study site contained more sulfate, sulfur reducing bacteria would contribute more to N fixation, considering that the sulfate addition resulted in an increase in N fixation of sulfur reducing bacteria (Fig. 4).
We also found that sulfur reducing bacteria in the sediment formed abou 5% of the total bacterial biomass (expressed as a proportion of sulfur reducing bacteria- PLFA biomarkers to total PLFA). Similarly, Kusel et al. (1999) detected 11 and 6% of sulfur reducing bacteria-PLFA biomarkers in the rhizosphere and unvegetated sediments of seagrass beds, respectively.
Sulfur reducing bacteria, even anaerobes, are able to tolerate exposure to oxygen indicating that they can experience periods of elevated oxygen tension, which can increase during the day when oxygen is produced by photosynthesis and transported to roots (Kusel et al. 1999). Sulfur reducing bacteria possess several oxygen defense strategies that allow survival in conditions of transient oxidative stress (Dolla et al. 2006; Miletto et al. 2008). This observation explains their occurrence and activity in the rhizosphere and sediment that drought-stressed wetlands.
Budget
A long-term objective of the nutrient addition experiment is to determine whether cyanobacterial N fixation can be substituted by heterotrophic N fixation. This question is of particular importance in a system like ours, where, due to P addition, cyanobacteria are eliminated by macrophytes with large N requirements. An N budget was constructed including potential heterotrophic N fixation under conditions of unlimited C supply as well as adjusted to actual C input from root exudates and plant litter decomposition. These estimations illustrate that if carbon and energy were not limiting, heterotrophic N fixation would provide enough new N to potentially cover macrophyte N requirements in P-amended plots. Assuming that C is unlimited, however, is unrealistic. Therefore scenario B, in which C supply it is estimated on the basis of measured exudation and decomposition rates, is a more appropriate estimate.
Although we use many assumptions to make these budgets, this is the first step in modeling the N balance. As we obtain more information, these budgets will become more realistic. At this point the following remain uncertain:
-
(1)
In terms of processes, only N fixation was taken into account and N mineralization and denitrification were not included. We did not measure these processes in the presented experiment, but data measured in 2005 and 2007 are available (Pivničková et al. 2010). Those data show that both N mineralization and denitrification was enhanced to a great extent in the P-amended plots. However, N gain by N mineralization and N loss by denitrification are by an order of magnitude lower than N gain by heterotrophic N fixation in the P-amended plots or by cyanobacterial N fixation in the control plots (Table 6). These results suggest that our estimates of N input using only N fixation data are neither highly underestimated nor overestimated.
-
(2)
The budget was constructed for the upper, organic and most biologically active sediment layer. Černá et al. (2009) present N fixation data also for the lower, mainly mineral sediment layer (to the depth of 30 cm) indicating that N fixation of this layer is lower but not negligible. We did not include N fixation of the lower sediment layer into the budget as more than 80% of roots are distributed in the upper layer (Černá et al. 2009; Snyder et al., in preparation).
-
(3)
Sediment N fixation might be underestimated as we assumed that the only source of C to the sediment is decomposition of dead plant material. We do not assume that the sediment N fixation could be affected directly by C flux from rhizosphere. It has been documented that up to 96% of released C from roots is incorporated into microbial biomass (Yevdokimov et al. 2007) and more than 80% of released C is used by rhizosphere microorganisms within 2 mm distance from the roots (Sauer et al. 2006). Given this observation, we believe that sediment N fixation was affected by higher C input primarily from the decomposition of plant litter rather than root exudates. It is important to note that both C sources are related. The exudation rate correlates with primary production and relates to the input of C to sediment via decomposition of dead plant material. The litter from the enhanced production due to P enrichment is also of better quality having lower C/N and C/P ratio (Rejmánková and Snyder 2008; Pivničková et al. 2010).
-
(4)
The slurry technique used in our experiments may provide equivocal results in C addition experiments because roots may be damaged during slurry preparation. This action may release and artificially increase the internal pools of labile C during incubation (Welsh et al. 1996b). In our experiments, potential N fixation was measured after the addition of glucose in excess (0.16 mg C cm−3). As documented by Černá et al. (2009), N fixation increased linearly with glucose additions until about a concentration of 0.3 mg C cm−3, and further additions had either null or even negative effect. On that account, labile C released from roots could increase N fixation twofold in maximum. This, coupled with the low proportion of root associated N fixation in overall heterotrophic N activity, leads us to believe that any labile C released by root damage could not result in a substantial overestimation of N fixation by root associated microflora.
-
(5)
The quantity and quality of C from exudates, and decomposing litter need to be more precise. Work on this objective is in progress.
Within the control plots, heterotrophic N fixation was very low and its contribution to total N fixation was negligible, illustrating that P-limited wetlands are dependent on autotrophic N fixation of cyanobacterial mats. Note that autotrophic N fixation provides the N required for macrophytes to sustain their growth (macrophyte N demand). In addition, N mineralization measured in these control plots was negative (Pivničková et al. 2010) indicating that N released during mineralization of organic matter was used by microbes to cover their N demand and as a result N was not available to higher plants, which is a common pattern for N limited ecosystems (Schimel and Bennett 2004). In this study, however, macrophyte tissue N in controls does not indicate any N limitation and is equal to or even higher than in P-amended plots (Rejmánková et al. 2008) and macrophyte N resorption efficiency is relatively low, suggesting that dominant macrophytes in controls are not limited by N.
With the exception of low salinity Typha plots, sediment N fixation in P-amended plots covered a lower proportion of macrophyte N demand compared to controls. However, evidence of macrophyte N limitation in P-amended plots is not found in their tissue N and C:N ratios.
In terms of N input to the wetland, root associated diazotrophs do not play a crucial role. The contribution of root associated N fixation to the total amount of N fixed, expressed per square meter, is much less than 1%. It would be inadequate, however, to depreciate the role of root associated diazotrophs as they also have other growth promoting traits (better development of roots due to production of plant-growth-factors, enhanced resistance to pathogens, and enhanced mineral uptake), which benefit the plant (Dobbelaere et al. 2003; Van Dommelen and Vanderleyden 2007).
Conclusions
We demonstrate that in tropical marshes of northern Belize:
-
(i)
P addition can substantially increase heterotrophic N fixation of both sediment and rhizosphere associated diazotrophs. Our long-term research data indicate that heterotrophic N fixation is induced by increased primary production of macrophytes.
-
(ii)
The contribution of root associated fixation to the total N fixation is small if expressed per square meter.
-
(iii)
Sulfate reducing diazotrophs are important component of N fixation even in low salinity marshes.
-
(iv)
Heterotrophic N fixation can substitute in part for autotrophic cyanobacterial N fixation when P limitation is alleviated.
References
Bagwell CE, Lovell CR (2000) Microdiversity of culturable diazotrophs from the rhizoplanes of the salt marsh grasses Spartina alterniflora and Juncus roemerianus. Microbial Ecol 39:128–136
Bergholz PW, Bagwell CE, Lovell CR (2001) Physiological diversity of rhizoplane diazotrophs of the saltmeadow cordgrass Spartina patens: Implications for host specific ecotypes. Microbial Ecol 42:466–473
Bevington PR, Robinson DK (2002) Data reduction and error analysis for the physical sciences, 3rd edn. McGraw-Hill, New York
Blaabjerg V, Finster K (1998) Sulphate reduction associated with roots and rhizomes of the marine macrophyte Zostera marina. Aquat Microb Ecol 15:311–314
Borbor-Cordova MJ, Boyer EW, McDowell WH, Hall CA (2006) Nitrogen and phosphorus budgets for a tropical watershed impacted by agricultural land use: Guayas Ecuador. Biogeochemistry 79:135–161
Boyle CD, Patriquin DG (1981) Carbon metabolism of Spartina-alterniflora loisel in relation to that of associated nitrogen-fixing bacteria. New Phytol 89:275–288
Bristow JM (1973) Nitrogen-fixation in rhizosphere of aquatic angiosperms. Plant Physiol 51:34
Bürgmann H, Meier S, Bunge M, Widmer F, Zeyer J (2005) Effects of model root exudates on structure and activity of a soil diazotroph community. Environ Microbiol 7:1711–1724
Capone DG (1982) Nitrogen fixation (acetylene reduction) by rhizosphere sediments of the Eelgrass Zostera marina. Mar Ecol Prog Ser 10:67–75
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and fresh-water sediments - contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33:725–749
Černá B, Rejmánková E, Snyder JM, Šantrůčková H (2009) Heterotrophic nitrogen fixation in oligotrophic tropical marshes: changes after phosphorus addition. Hydrobiologia 627:55–65
Dakora FD, Drake BG (2000) Elevated CO2 stimulates associative N2 fixation in a C-3 plant of the Chesapeake Bay wetland. Plant Cell Environ 23:943–953
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth-promoting effects of diazotrophs in the rhizosphere. Crit Rev Plant Sci 22:107–149
Dodla SK, Wang JJ, Delaune RD, Breitenbeck G (2009) Carbon gas production under different electron acceptors in a freshwater marsh soil. Chemosphere 76:517–522
Dolla A, Fournier M, Dermoun Z (2006) Oxygen defense in sulfate-reducing bacteria. J Biotechnol 126:87–100
Eskew DL, Eaglesham ARJ, App AA (1981) Heterotrophic 15N2 fixation and distribution of newly fixed nitrogen in a rice-flooded soil system. Plant Physiol 68:48–52
Frostegård A, Tunlid A, Bååth E (1993) Phospholipid fatty-acid composition biomass and activity of microbial communities from 2 soil types experimentally exposed to different heavy-metals. Appl Environ Microb 59:3605–3617
Herbert RA (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23:563–590
Howarth R, Marino WR, Lane J, Cole JJ (1988) Nitrogen-fixation in fresh-water estuarine and marine ecosystems. 1. Rates and importance. Limnol Oceanogr 33:669–687
Inglett PW, Reddy KR, McCormick PV (2004) Periphyton chemistry and nitrogenase activity in a northern Everglades ecosystem. Biogeochemistry 67:213–233
Inglett PW, D’Angelo EM, Reddy KR, McCormick PV, Hagerthey SE (2009) Periphyton nitrogenase activity as an indicator of wetland eutrophication: spatial patterns and response to phosphorus dosing in a northern Everglades ecosystem. Wetlands Ecol Manag 17:131–144
Johnson S, Rejmánková E (2005) Impacts of land use on nutrient distribution and vegetation composition of freshwater wetlands in Northern Belize. Wetlands 25:89–100
King RB, Baillie IC, Abell TMB, Dunsmore JR, Gray DA, Pratt JH, Versey HR, Wright ACS, Zisman SA (1992) Land resources assessment of Northern Belize. Nat Resour Inst Bull 43:513
Kohring LL, Ringelberg DB, Devereux R, Stahl DA, White DC (1994) Comparison of phylogenetic relationships based on phospholipid fatty acid profiles and ribosomal RNA sequence similarities among dissimilatory sulphate-reducing bacteria. FEMS Microbiol Lett 119:303–308
Koretsky CM, Van Cappellen P, DiChristina TJ, Kostkad JE, Lowe KL, Moore CM, Roychoudhury AR, Viollier E (2005) Salt marsh pore water geochemistry does not correlate with microbial community structure. Estuar Coast Shelf Sci 62:233–251
Kusel K, Pinkart HC, Drake HL, Devereux R (1999) Acetogenic and sulfate-reducing bacteria inhabiting the rhizoplane and deep cortex cells of the sea grass Halodule wrightii. Appl Environ Microbiol 65:5117–5123
Lee RY, Joye SB (2006) Seasonal patterns of nitrogen fixation and denitrification in oceanic mangrove habitats. Mar Ecol Prog Ser 307:127–141
Londry KL, Jahnke LL, DesMarais DJ (2004) Stable isotope ratios of lipid biomarkers of sulfate-reducing bacteria. Appl Environ Microbiol 70:745–751
McDowell WH (1998) Internal nutrient fluxes in a Puerto Rican rain forest. J Trop Ecol 14:521–536
Miletto M, Loy A, Antheunisse AM, Loeb R, Bodelier PLE, Laanbroek HJ (2008) Biogeography of sulfate-reducing prokaryotes in river floodplains. FEMS Microbiol Ecol 64:395–406
Montoya JP, Voss M, Kähler P, Capone DG (1996) A simple, high-precision, high-sensitivity tracer assay for N-2 fixation. Appl Environ Microbiol 62:986–993
Moseman SM (2007) Opposite diel patterns of nitrogen fixation associated with salt marsh plant species (Spartina foliosa and Salicornia virginica) in southern California. Mar Ecol Evol Perspect 28:276–287
Nielsen LB, Finster K, Welsh DT, Donelly A, Herbert RA, de Wit R, Lomstein BA (2001) Sulphate reduction and nitrogen fixation rates associated with roots rhizomes and sediments from Zostera noltii and Spartina maritima meadows. Environ Microb 3:63–71
Oremland RS, Capone DG (1988) Use of specific inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol 10:285–383
Pelegri SP, Twilley RR (1998) Heterotrophic nitrogen fixation (acetylene reduction) during leaf-litter decomposition of two mangrove species from south Florida, USA. Mar Biol 131:53–61
Pivničková B, Rejmánková E, Snyder JM, Šantrůčková H (2010) Heterotrophic microbial activities and physiological status in tropical marsh sediments of different salinities: the effect of phosphorus addition and plant species. Plant Soil. doi:10.1007/s11104-010-0439-6
Postgate JR, Kent HM (1985) Diazotrophy within Desulfovibrio. J Gen Microbiol 131:2119–2122
Reed SC, Cleveland CC, Townsend AR (2007) Controls over leaf litter and soil nitrogen fixation in two lowland tropical rain forests. Biotropica 39:585–592
Rejmánková E (2001) Effect of experimental phosphorus enrichment on oligotrophic tropical marshes in Belize, Central America. Plant Soil 236:33–53
Rejmánková E, Houdková K (2006) Wetland plant decomposition under different nutrient conditions: what is more important litter quality or site quality? Biogeochemistry 80:245–262
Rejmánková E, Komárková J (2000) A function of cyanobacterial mats in phosphorus-limited tropical wetlands. Hydrobiologia 431:135–153
Rejmánková E, Komárková J (2005) Response of cyanobacterial mats to nutrient and salinity changes. Aquat Bot 83:87–107
Rejmánková E, Snyder JM (2008) Emergent macrophytes in phosphorus limited marshes: do phosphorus usage strategies change after nutrient addition? Plant Soil 313:141–153
Rejmánková E, Komárková J, Rejmánek M (2004) Delta N-15 as an indicator of N-2-fixation by cyanobacterial mats in tropical marshes. Biogeochemistry 67:353–368
Rejmánková E, Macek P, Epps K (2008) Wetland ecosystem changes after three years of phosphorus addition. Wetlands 28:914–927
Riederer-Henderson M, Wilson PW (1970) Nitrogen fixation by sulphate-reducing bacteria. J Gen Microbiol 61:27–31
Roger PA (1995) Biological N-2-fixation and its management in wetland rice cultivation. Fert Res 42:261–276
Roger PA, Ladha JK (1992) Biological N2 fixation in wetland rice fields—estimation and contribution to nitrogen-balance. Plant Soil 141:41–55
Šantrůčková H, Černá B, Snyder J, Rejmánková E (2009) Nutrient enrichment in tropical wetlands: shifts in autotrophic versus heterotrophic nitrogen fixation. In: Working Papers of the Finnish Forest Research Institute, vol 128, p 349
Sauer D, Kuzyakov Y, Stahr K (2006) Spatial distribution of root exudates of five plant species as assessed by 14C labeling. J Plant Nutr Soil Sci 169:360–362
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Scott JT, Doyle RD, Filstrup CT (2005) Periphyton nutrient limitation and nitrogen fixation potential along a wetland nutrient-depletion gradient. Wetlands 25:439–448
Scott JT, Doyle RD, Back JA, Dworkin SI (2007) The role of N2 fixation in alleviating N limitation in wetland meta-phyton: enzymatic isotopic and elemental evidence. Biogeochemistry 84:207–218
Scott JT, Doyle RD, Prochnow SJ, White JD (2008) Are watershed and lacustrine controls on planktonic N2 fixation hierarchically structured? Ecol Appl 18:805–819
Steppe TF, Paerl HW (2002) N2 fixation by sulfate-reducing bacteria in a marine intertidal microbial mat. Aquat Microb Ecol 28:1–12
Taylor J, Parkes RJ (1983) The cellular fatty acids of the sulphate-reducing bacteria Desulfobacter sp. Desulfobulbus sp. and Desulfovibrio desulfuricans. J Gen Microbiol 129:3303–3309
Tjepkema JD, Evans HJ (1976) Nitrogen-fixation associated with Juncus-balticus and other plants of Oregon wetlands. Soil Biol Biochem 8:505–509
Tyler AC, Mastronicola TA, McGlathery KJ (2003) Nitrogen fixation and nitrogen limitation of primary production along a natural marsh chronosequence. Oecologia 136:431–438
Van Dommelen A, Vanderleyden J (2007) Associative nitrogen fixation. In: Bothe H, Ferguson SJ, Newton WE (eds) Biology of nitrogen cycle. Elsevier B.V., Amsterdam, p 452
Welsh DT, Bourgues S, deWit R, Herbert RA (1996a) Seasonal variations in nitrogen-fixation (acetylene reduction) and sulphate-reduction rates in the rhizosphere of Zostera noltii: nitrogen fixation by sulphate reducing bacteria. Mar Biol 125:619–628
Welsh DT, Wellsbury P, Bourgues S, de Wit R, Herbert RA (1996b) Relationship between porewater organic carbon content sulphate reduction and nitrogen fixation (acetylene reduction) in the rhizosphere of Zostera noltii. Hydrobiologia 329:175–183
Yevdokimov IV, Ruser R, Buegger F, Marx M, Munch JC (2007) Interaction between rhizosphere microorganisms and plant roots: 13C fluxes in the rhizosphere after pulse labeling. Soil Biol 40:766–774
Zehr JP, Montoya JP (2007) Measuring N2 fixation in the field. In: Bothe H, Ferguson SJ, Newton WE (eds) Biology of nitrogen cycle. Elsevier B.V., Amsterdam, pp 452
Zelles L, Bai QY, Beck T, Beese F (1992) Signature fattyacids in phospholipids and lipopolysacharides as indicators of microbial biomass and community structure in agriculture soils. Soil Biol Biochem 24:317–323
Acknowledgments
We would like to thank Ireneo Briceno, Russell King for their assistance in the field, Emily Carlson, Tereza Říhová, Daniel Vaněk and Tomáš Picek for laboratory assistance. This research was supported by the following grants: NSF # 0089211 to E. R.; ME 912, and MSM # 600 766 5801 to H. Š.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Šantrůčková, H., Rejmánková, E., Pivničková, B. et al. Nutrient enrichment in tropical wetlands: shifts from autotrophic to heterotrophic nitrogen fixation. Biogeochemistry 101, 295–310 (2010). https://doi.org/10.1007/s10533-010-9479-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9479-5