Abstract
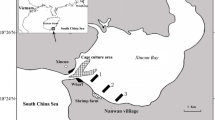
Organic matter oxidation represents a transfer of elements to inorganic nutrients that support biological productivity and food web processes. Therefore, quantification of the controls of organic matter mineralization is crucial to understanding the carbon cycle and biogeochemical dynamics in coastal marine environments. We investigated the rates and pathways of anaerobic carbon (C) oxidation in an unvegetated mud flat (UMF) and a vegetated mud flat (VMF) of the Ganghwa intertidal zone of the macro-tidal Han River estuary, Yellow Sea. Analyses of geochemical constituents revealed relatively oxidized conditions and high reactive Fe(III) concentrations (40–100 μmol cm−3) in the sediments. A pronounced depth stratification in Fe(III) was observed at the VMF site likely due to the lower number of infaunal burrows along with dense root formation by the macrophytes, Suaeda japonica. Depth-integrated rates of anaerobic C mineralization as well as sulfate- and Fe(III) reduction at the VMF were consistently higher than those at the UMF, likely driven by the dense vegetation that supplied organic C substrates and electron acceptors to the rhizosphere. Sediment inventories revealed that solid Fe(III) was up to 17 times more abundant than pore water sulfate, and direct rate measurements showed that microbial Fe(III) reduction comprised an equal or larger percentage of C oxidation (36–66 %) in comparison to sulfate reduction (36–40 %) at both sites studied. Time-course experiments indicated that sulfate reduction rates were likely underestimated, especially in the VMF rhizosphere, due to the reoxidation of reduced S in the presence of high Fe(III). The high rates of C mineralization suggest that the Ganghwa intertidal mud flats are a significant sink against the external loading of organic compounds, and organic matter mineralization is enhanced by chemical exchange regulated by extreme tidal flushing and macro-microorganisms interactions.







Similar content being viewed by others
References
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114:331–345. doi:10.1016/0009-2541(94)90062-0
Aller RC (2001) Transport and reactions in the bioirrigated zone. In: Boudreau BP, Jorgensen BB (eds) The benthic boundary layer: transport processes and biogeochemistry. Oxford University Press, Oxford, pp 269–301
Aller RC, Mackin JE, Cox RT Jr (1996) Diagenesis of Fe and S in Amazon inner shelf muds: apparent dominance of fe reduction and implications for the genesis of ironstones. Cont Shelf Res 6:263–289. doi:10.1016/0278-4343(86)90064-6
Alongi DM (1998) Coastal Ecosystem Processes. CRC Press, Boca Raton, p 419
An S (2005) Seasonal variations of sediment oxygen demand and denitrification in Kanghwa tidal flat sediments. (The Sea). J Kor Soc Oceanogr 10:47–55 In Korean
Banta GT, Holmer M, Jensen M, Kristensen E (1999) Effects of two polychaete worms, Nereis diversicolor and Arenicola marina, on aerobic and anaerobic decomposition in a sandy marine sediment. Aquat Microb Ecol 19:189–204. doi:10.3354/ame019189
Bertness MD (1985) Fiddler crab regulation of Spartina alterniflora production on a new England salt marsh. Ecology 66:1042–1055. doi:10.2307/1940564
Boudreau BP, Jørgensen BB (2001) The benthic boundary layer: transport processes and biogeochemistry. Oxford Univ Press, Oxford
Bowman JP, McCuaig RD (2003) Biodiversity, community structural shifts, and biogeography of prokaryotes within Antarctic continental shelf sediments. Appl Environ Microbiol 69:2463–2483. doi:10.1128/AEM.69.5.2463-2483.2003
Canfield DE, Thamdrup B, Kristensen E (2005) Aquatic geomicrobiol. Elsevier, Amsterdam, p 640
Capone DG, Kiene R (1988) Comparison of microbial dynamics in marine and freshwater sediments: Contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33:725–749
Cline JD (1969) Spectrophotometric determinations of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Dollhopf SL, Hyun J-H, Smith AC, Adams HJ, O’Brien S, Kostka JE (2005) Quantification of ammonia-oxidizing bacteria and controls of nitrification in saltmarsh sediments. Appl Environ Microbiol 71:240–246. doi:10.1128/AEM.71.1.240-246.2005
Emerson D, Weiss JV, Megonigal JP (1999) Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl Environ Microbiol 65:2758–2761
Fossing H, Jørgensen BB (1989) Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochem 8:205–222. doi:10.1007/BF00002889
Furukawa Y, Smith AC, Kostka JE, Watkins J, Alexander CR (2004) Quantification of macrobenthic effects on diagenesis using a multicomponent inverse model in salt marsh sediments. Limnol Oceanogr 49:2058–2072
Giblin AE, Hopkins CS, Tucker J (1997) Benthic metabolism and nutrient cycling in Boston harbor, Massachusetts. Estuaries 20:346–364. doi:10.2307/1352349
Gribsholt B, Kristensen E (2002) Effects of bioturbation and plant roots on salt marsh biogeochemistry: a mesocosm study. Mar Ecol Prog Ser 241:71–87. doi:10.3354/meps241071
Gribsholt B, Kristensen E (2003) Benthic metabolism and sulfur cycling along an inundation gradient in a tidal Spartina anglica salt marsh. Limnol Oceanogr 48:2151–2162
Gribsholt B, Kostka JE, Kristensen E (2003) Impact of fiddler crabs and plant roots on sediment biogeochemistry in a Georgia slatmarsh. Mar Ecol Prog Ser 259:237–251. doi:10.3354/meps259237
Hall PO, Aller RC (1992) Rapid small-volume, flow injection analysis for CO2 and NH4 + in marine and freshwaters. Limnol Oceanogr 37:113–119
Hansen K, King GM, Kristensen E (1996) Impact of the soft-shell clam Mya arenaria on sulfate reduction in an intertidal sediment. Aquat Microb Ecol 10:181–194. doi:10.3354/ame010181
Hines M, Jones GE (1985) Microbial biogeochemistry and bioturbation in the sediments of Great Bay, New Hampshire. Estuar Coast Shelf Sci 20:729–742. doi:10.1016/0272-7714(85)90029-0
Hines ME, Knollmeye SL, Tugel JB (1989) Sulfate reduction and other sedimentary biogeochemistry in northern New England salt marsh. Limnol Oceanogr 34:578–590
Hines ME, Evans RS, Genthner BRS, Willis SG, Friedman S, Rooney-Varga JN, Devereux R (1999) Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol 65:2209–2216
Holmer M, Forbes VE, Forbes TL (1997) Impact of the polychaete Capitella sp. I on microbial activity in an organic-rich marine sediment contaminated with the polycyclic aromatic hydrocarbon fluoranthene. Mar Biol (Berl) 128:679–688. doi:10.1007/s002270050135
Holmer M, Andersen FØ, Holmboe N, Kristensen E, Thongtham N (1999) Transformation and exchange processes in the Bangrong mangrove forest-seagrass bed system, Thailand. Seasonal and spatial variations in benthic metabolism and sulfur biogeochemistry. Aquat Microb Ecol 20:203–212. doi:10.3354/ame020203
Holmer M, Andersen FØ, Nielsen SL, Boschker HTS (2001) The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aquat Bot 71:1–17. doi:10.1016/S0304-3770(01)00170-X
Howarth RW (1993) Microbial processes in salt-marsh sediments. In: Ford TE (ed) Aquatic microbiology: An ecological approach. Blackwell, Cambridge, pp 239–259
Howes BL, Dacey JWH, King GM (1984) Carbon flow through oxygen and sulfate reduction pathways in salt marsh sediments. Limnol Oceanogr 29:1037–1051
Hyun J-H, Choi JK, Chung JH, Yang EJ, Kim MK (1999a) Tidally induced changes in bacterial growth and viability in the macrotidal Han River estuary, Yellow Sea. Estuar Coast Shelf Sci 48:143–153. doi:10.1006/ecss.1998.0421
Hyun J-H, Chung KH, Park YC, Choi JK (1999b) Variations in ammonium removal rate withtidal stat in the macrotidal Han River estuary: potential role of nitrification. (The Sea). J Kor Soc Oceanogr 4:33–39
Hyun J-H, Mok JS, Cho HY, Cho BC, Choi JK (2004) Anaerobic mineralization of organic matter and sulfate reduction in summer at Ganghwa intertidal flat, Korea. J Korean Wetlands Soc 6:132 In Korean
Hyun J-H, Smith AC, Kostka JE (2007) Relative contributions of sulfate- and iron(III) reduction to organic matter mineralization and process controls in contrasting habitats of the Georgia saltmarsh. Appl Geochem 22:2637–2651. doi:10.1016/j.apgeochem.2007.06.005
Isaksen MF, Finster K (1996) Sulphate rduction in the root zone of the seagrass Zostera noltii on the intertidal flats of a coastal lagoon (Arcachon, France). Mar Ecol Prog Ser 137:187–194. doi:10.3354/meps137187
Jensen HS, Mortensen PB, Andersen FØ, Rasmussen E, Jensen A (1995) Phosphorus cycling in a coastal marine sediment, Aarhus bay, Denmark. Limnol Oceanogr 40:908–917
Jensen MM, Thamdrup B, Rysgaard S, Holmer M, Fossing H (2003) Rates and regulation of microbial iron reduction in sediments of the Baltic-North Sea transition. Biogeochem 65:295–317. doi:10.1023/A:1026261303494
Jørgensen BB (1978) A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments, 1. Measurement with radiotracer techniques. Geomicrobiol J 1:11–28
Jørgensen BB (1982) Mineralization of organic matter in the sea bed - the role of sulphate reduction. Nature 96:643–645. doi:10.1038/296643a0
Jørgensen BB (1994) Sulfate reduction and thiosulfate transformations in a cyanobacterial mats during diel oxygen cycle. FEMS Microbiol Ecol 13:303–312. doi:10.1016/0168-6496(94)90068-X
Jørgensen BB (2000) Bacteria and marine biogeochemistry. In: Schulz HD, Zabel M (eds) Marine geochemistry. Springer, Berlin, pp 173–207
Kim DS (2006) Estimation of oxygen consumption rates using an oxygen microelectrode in Ganghwa intertidal flat. Ocean Polar Res 28:119–127 In Korean
King GM (1988) Patterns of sulfate reduction and the sulfur cycle in a South Carolina salt marsh. Limnol Oceanogr 33:376–390
King GM, Garey MA (1999) Ferric iron reduction by bacteria associated with the roots of freshwater and marine macrophytes. Appl Environ Microbiol 65:4393–4398
King GM, Klug MJ, Wiegert RG, Chalmers AG (1982) Relation of soil water movement and sulfide concentration to Spartina alterniflora production in a Georgia Salt Marsh. Science 218:61–63
Koo BJ, Kwon KK, Hyun J-H (2005) The sediment-water interface increment due to the complex burrows of macrofauna in a tidal flat. Ocean Sci J 40:221–227
Koo BJ, Kwon KK, Hyun J-H (2007) Effect of environmental conditions on variation in the sediment-water interface created by complex macrofaunal burrows on a tidal flat. J Sea Res 58:302–312. doi:10.1016/j.seares.2007.07.002
Koretsky CM, Moore CM, Lowe KL, Meile C, Dichristina TJ, Van Cappellen P (2003) Seasonal oscillation of microbial iron and sulfate reduction in saltmarsh sediments (Sapelo Island, GA, USA). Biogeochem 64:179–203. doi:10.1023/A:1024940132078
Koretsky CM, Van Cappellen P, DiChristina TJ, Kostka JE, Lowe KL, Moore CM, Roychoudhury AN, Viollier E (2005) Salt marsh porewater geochemistry does not correlate with microbial community structure. Estuar Coast Shelf Sci 62:233–251. doi:10.1016/j.ecss.2004.09.001
Koretsky CM, Haveman M, Cuellar A, Beuving L, Shattuck T, Wagner M (2008a) Influence of Spartina and Juncus on saltmarsh sediments. I. pore water geochemistry. Chem Geol 255:87–99. doi:10.1016/j.chemgeo.2008.06.013
Koretsky CM, Cuellar A, Haveman M, Beuving L, Shattuck T, Wagner M (2008b) Influence of Spartina and Juncus on saltmarsh sediments. II. Trace element geochemistry. Chem Geol 255:100–113. doi:10.1016/j.chemgeo.2008.06.014
Kostka JE, Gribsholt B, Petrie E, Dalton D, Skelton H, Kristensen E (2002a) The rates and pathways of carbon oxidation in bioturbated saltmarsh sediments. Limnol Oceanogr 47:230–240
Kostka JE, Roychoudhury A, Van Cappellen P (2002b) Rates and controls of anaerobic microbial respiration across spatial and temporal gradients in saltmarsh sediments. Biogeochem 60:49–76. doi:10.1023/A:1016525216426
Kristensen E (2001) Impact of polychaetes (Nereis spp. and Arenicola marina) on carbon biogeochemistry in coastal marine sediment. A review. Geochem Trans 2:92–104. doi:10.1186/1467-4866-2-92
Kristensen E, Kostka JE (2005) Macrofaunal burrows and irrigation in marine sediment: microbiological and biogeochemical interactions. In: Kristensen E, Haese RR, Kostka JE (eds) Interaction between macro- and microorganisms in marine sediments, vol 60. American Geophysical Union, Washington, DC, pp 125–157
Kristensen E, Andersen FØ, Holmboe N, Holmer M, Thongtham N (2000) Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquat Microb Ecol 22:199–213. doi:10.3354/ame022199
Lee K, Kim I (2006) Status and breeding biology of black-faced spoonbills in Korea. Proceedings: Keeping Asia’s spoonbills airborne. International Symposium on research and conservation of the black faced spoonbill, Hong Kong, pp 56–62
Leven LA, Talley TS (2000) Influence on vegetation and abiotic environmental factors on salt marsh invertebrates. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer Academic Publishers, Dordrecht, pp 661–707
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Phillips EJP (1987) Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol 53:2636–2641
Lowe KL, DiChristina TJ, Roychoudhury AN, Van Cappellen P (2000) Microbiological and geochemical characterization of microbial Fe(III) reduction in salt marsh sediments. Geomicrobiol J 17:163–178. doi:10.1080/01490450050023836
Lustwerk RL, Burdige DJ (1995) Elimination of dissolved sulfide interference in the flow injection dermination of ΣCO2 by addition of molybdate. Limnol Oceanogr 40:1011–1012
Min BM (1998) Vegetation on the west coast of Korea. Ocean Res 20:167–178
Moeslund L, Thamdrup B, Jørgensen BB (1994) Sulfur and iron cycling in a coastal sediment: radiotracer studies and seasonal dynamics. Biogeochem 27:129–152
MOMAF (1999) Our coastal wetland, tidal flat ‘Getbul’. Ministry of Maritime Affairs and Fisheries. p 38
Neubauer SC, Valentine SK, Kim G, Megonigal JP (2005) Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86:3334–3344. doi:10.1890/04-1951
Nielsen T, Andersen FØ (2003) Phosphorus dynamics during decomposition of mangrove (Rhizophora apiculata) leaves in sediments. J Exp Mar Biol Ecol 293:73–88. doi:10.1016/S0022-0981(03)00200-4
Nielsen OI, Kristensen E, Holmer M (2003) Impact of Arenicola marina (polychaeta) on sediment sulfur dynamics. Aquat Microb Ecol 33:95–105. doi:10.3354/ame033095
OHA (1978) Technical reports of hydrographic office of republic of Korea. Office of Hydrographic Affairs. Pub No 1101
Odum EP (2000) Tidal marshes as outwelling/pulsing systems. In: Weinstein MP, Kreeger DA (eds) Concepts and controversies in tidal marsh ecology. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 3–7
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawaer analysis. Pergamon Press, Oxford, p 173
Phillips EJP, Lovley DR (1987) Determination of Fe(III) and Fe(II) in oxalate extracts of sediment. Soil Sci Soc Am J 51:938–941
Pomeroy LR, Wiegert RG (1981) The ecology of a salt marsh. Springer-Verlag, New York, p 271
Postma D, Jakobsen R (1996) Redox zonation: equilibrium constraints on the Fe(III)/SO42- reduction interface. Geochim Cosmochim Acta 60:3169–3175. doi:10.1016/0016-7037(96)00156-1
Poulton SW, Raiswell R (2002) The low-temperature geochemical cycle of iron: From continental fluxes to marine sediment deposition. Am J Sci 302:774–805. doi:10.2475/ajs.302.9.774
Ravenschlag K, Sahm K, Pernthaler J, Amann R (1999) High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol 65:3982–3989
Ravenschlag K, Sahm K, Knoblauch C, Jørgensen BB, Amann R (2000) Community structure, cellular rRNA content, and activity of sulfate-reducing bacteria in marine Arctic sediments. Appl Environ Microbiol 66:3592–3602. doi:10.1128/AEM.66.8.3592-3602.2000
Roden EE, Wetzel RG (1996) Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr 41:1733–1748
Rooney-Varga JN, Devereux R, Evans R, Hines ME (1997) Seasonal changes in the relative abundance of uncultivated sulfate-reducing bacteria in salt marsh sediment and in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol 63:3895–3901
Smith AC, Kostka JE, Devereux R, Yates DF (2004) Seasonal composition and activity of sulfate-reducing prokaryotic communities in seagrass bed sediments. Aquat Microb Ecol 37:183–195. doi:10.3354/ame037183
Sobolev D, Roden EE (2001) Suboxic deposition of ferric iron by bacteria in opposing gradients of Fe(II) and oxygen at circumneutral pH. Appl Environ Microbiol 67:1328–1334. doi:10.1128/AEM.67.3.1328-1334.2001
Stookey LL (1970) Ferrozine–a new spectrophotometric reagent for iron. Anal Chem 42:245–252. doi:10.1021/ac60289a016
Thamdrup B (2000) Bacterial manganese and iron reduction in aquatic sediments. Adv Microb Ecol 16:41–84
Thamdrup B, Canfield DE (1996) Pathways of carbon oxidation in continental margin sediments off central Chile. Limnol Oceanogr 41:1629–1650
Thamdrup B, Canfield DE (2000) Benthic respiration in aquatic sediments. In: Jackson RB, Sala OE, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 86–103
Weiss JV, Emerson D, Backer SM, Megonigal JP (2003) Enumeration of Fe(II)-oxidizing and Fe(III)-reducing bacteria in the root zone of wetland plants: implications for a rhizosphere iron cycle. Biogeochem 64:77–96. doi:10.1023/A:1024953027726
White DS, Howes BL (1994) Long-term 15N-nitrogen retention in the vegetated sediments of a New England salt marsh. Limnol Oceanogr 39:1878–1892
Acknowledgments
This study was supported by the Basic Research Program of Korea Ocean R&D Institute (PE 98104), Development of Utilization Technique for Tidal Energy (PM 51700) funded by the Korean Ministry of Land, Transport and Maritime Affairs, and partly by the internal research fund of Hanyang University (HY-2006-N). The authors thank two anonymous reviewers for their comments that improved the earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyun, JH., Mok, JS., Cho, HY. et al. Rapid organic matter mineralization coupled to iron cycling in intertidal mud flats of the Han River estuary, Yellow Sea. Biogeochemistry 92, 231–245 (2009). https://doi.org/10.1007/s10533-009-9287-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-009-9287-y