Abstract
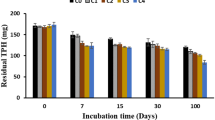
Environmental microbial communities are key players in the bioremediation of hydrocarbon pollutants. Here we assessed changes in bacterial abundance and diversity during the degradation of Tunisian Zarzatine oil by four indigenous bacterial consortia enriched from a petroleum station soil, a refinery reservoir soil, a harbor sediment and seawater. The four consortia were found to efficiently degrade up to 92.0% of total petroleum hydrocarbons after 2 months of incubation. Illumina 16S rRNA gene sequencing revealed that the consortia enriched from soil and sediments were dominated by species belonging to Pseudomonas and Acinetobacter genera, while in the seawater-derived consortia Dietzia, Fusobacterium and Mycoplana emerged as dominant genera. We identified a number of species whose relative abundances bloomed from small to high percentages: Dietzia daqingensis in the seawater microcosms, and three OTUs classified as Acinetobacter venetianus in all two soils and sediment derived microcosms. Functional analyses on degrading genes were conducted by comparing PCR results of the degrading genes alkB, ndoB, cat23, xylA and nidA1 with inferences obtained by PICRUSt analysis of 16S amplicon data: the two data sets were partly in agreement and suggest a relationship between the catabolic genes detected and the rate of biodegradation obtained. The work provides detailed insights about the modulation of bacterial communities involved in petroleum biodegradation and can provide useful information for in situ bioremediation of oil-related pollution.





Similar content being viewed by others
References
Abbasian F, Lockington R, Megharaj M, Naidu R (2016) A review on the genetics of aliphatic and aromatic hydrocarbon degradation. Appl Biochem Biotech 178:224–250
Aburto-Medina A, Adetutu EM, Aleer S, Weber J, Patil SS, Sheppard PJ, Ball AS, Juhasz AL (2012) Comparison of indigenous and exogenous microbial populations during slurry phase biodegradation of long-term hydrocarbon-contaminated soil. Biodegradation 23:813–822
Alvarez HM, Souto MF, Viale A, Pucci OH (2001) Biosynthesis of fatty acids and triacylglycerols by 2,6,10,14-tetramethyl pentadecane-grown cells of Nocardia globerula 432. FEMS Microbiol Lett 200:195–200
Arenghi FL, Berlanda D, Galli E, Sello G, Barbieri P (2001) Organization and regulation of metaCleavage pathway genes for toluene and o-xylene derivative degradation in Pseudomonas stutzeri OX1. Appl Environ Microb 67:3304–3308
Bacosa H, Suto K, Inoue C (2010) Preferential degradation of aromatic hydrocarbons in kerosene by a microbial consortium. Int Biodeter Biodegr 64:702–710
Baldi F, IvoŠević N, Minacci A, Pepi M, Fani R, Svetličić V, ZŬtić V (1999) Adhesion of Acinetobacter venetianus to diesel fuel droplets studied with in situ electrochemical and molecular probes. Appl Environ Microb 65:2041–2048
Bassi D, Puglisi E, Cocconcelli PS (2015) Understanding the bacterial communities of hard cheese with blowing defect. Food Microbiol 52:106–118
Bogan B, Lahner L, Sullivan W, Paterek J (2003) Degradation of straight-chain aliphatic and high-molecular-weight polycyclic aromatic hydrocarbons by a strain of Mycobacterium austroafricanum. J Appl Microbiol 94:230–239
Brezna B, Khan AA, Cerniglia CE (2003) Molecular characterization of dioxygenases from polycyclic aromatic hydrocarbon-degrading Mycobacterium spp. FEMS Microbiol Lett 223:177–183
Bundy J, Paton G, Campbell C (2002) Microbial communities in different soil types do not converge after diesel contamination. J Appl Microbiol 92:276–288
Chaerun SK, Tazaki K, Asada R, Kogure K (2005) Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: implications for bioremediation. Clay Miner 40:105–114
Cheema S, Lavania M, Lal B (2015) Impact of petroleum hydrocarbon contamination on the indigenous soil microbial community. Ann Microbiol 65:359–369
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200
El Amrani A, Dumas A-S, Wick LY, Yergeau E, Berthomé R (2015) “Omics” insights into PAH degradation toward improved green remediation biotechnologies. Environ Sci Technol 49:11281–11291
Ferrero M, Llobet-Brossa E, Lalucat J, Garcia-Valdes E, Rossello-Mora R, Bosch R (2002) Coexistence of two distinct copies of naphthalene degradation genes in Pseudomonas strains isolated from the western Mediterranean region. Appl Environ Microbiol 68(2):957–962
Fuentes S, Barra B, Caporaso JG, Seeger M (2016) From rare to dominant: a fine-tuned soil bacterial bloom during petroleum hydrocarbon bioremediation. Appl Environ Microb 82:888–896
Gallego S, Vila J, Tauler M, Nieto JM, Breugelmans P, Springael D, Grifoll M (2014) Community structure and PAH ring-hydroxylating dioxygenase genes of a marine pyrene-degrading microbial consortium. Biodegradation 25:543–556
Gihring TM, Green SJ, Schadt CW (2012) Massively parallel rRNA sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol 14:285–290
Good IJ (1953) The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264
Guibert LM, Loviso CL, Borglin S, Jansson JK, Dionisi HM, Lozada M (2016) Diverse bacterial groups contribute to the alkane degradation potential of chronically polluted subantarctic coastal sediments. Microb Ecol 71(1):100–112
Harayama S, Kishira H, Kasai Y, Shutsubo K (1999) Petroleum biodegradation in marine environments. J Mol Microb Biotech 1:63–70
Harayama S, Kasai Y, Hara A (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotech 15:205–214
He P, Li L, Liu J, Bau Y, Fang X (2016) Diversity and distribution of catechol 2,3-dioxygenase genes in subsurface sediments of the Bohai sea. FEMS Microbiol Lett 363:fnw068
Hendrickx B, Dejonghe W, Faber F, Boënne W, Bastiaens L, Verstraete W, Top EM, Springael D (2006) PCR-DGGE method to assess the diversity of BTEX mono-oxygenase genes at contaminated sites. FEMS Microbiol Ecol 55:262–273
Hentati O, Lachhab R, Ayadi M, Ksibi M (2013) Toxicity assessment for petroleum-contaminated soil using terrestrial invertebrates and plant bioassays. Environ Monit Assess 185:2989–2998
Hesham AE-L, Mawad AM, Mostafa YM, Shoreit A (2014) Biodegradation ability and catabolic genes of petroleum-degrading Sphingomonas koreensis strain ASU-06 isolated from Egyptian oily soil. Biomed Res Int. https://doi.org/10.1155/2014/127674
Kaplan CW, Kitts CL (2004) Bacterial succession in a petroleum land treatment unit. Appl Environ Microb 70:1777–1786
Khan AA, Wang R-F, Cao W-W, Doerge DR, Wennerstrom D, Cerniglia CE (2001) Molecular cloning, nucleotide sequence, and expression of genes encoding a polycyclic aromatic ring dioxygenase from Mycobacterium sp. strain PYR-1. Appl Environ Microb 67:3577–3585
Klankeo P, Nopcharoenkul W, Pinyakong O (2009) Two novel pyrene-degrading Diaphorobacter sp. and Pseudoxanthomonas sp. isolated from soil. J Biosci Bioeng 108:488–495
Kloos K, Munch JC, Schloter M (2006) A new method for the detection of alkane-monooxygenase homologous genes (alkB) in soils based on PCR-hybridization. J Microbiol Meth 66:486–496
Koo H, Mojib N, Huang JP, Donahoe RJ, Bej AK (2015) Bacterial community shift in the coastal Gulf of Mexico salt-marsh sediment microcosm in vitro following exposure to the Mississippi Canyon Block 252 oil (MC252). 3. Biotech 5:379–392
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the Deepwater Horizon oil spill. Appl Environ Microb 77:7962–7974
Kulik N, Goi A, Trapido M, Tuhkanen T (2006) Degradation of polycyclic aromatic hydrocarbons by combined chemical pre-oxidation and bioremediation in creosote contaminated soil. J Environ Manag 78:382–391
Lai Q, Wang L, Liu Y, Fu Y, Zhong H, Wang B, Chen L, Wang J, Sun F, Shao Z (2011) Alcanivorax pacificus sp. nov., isolated from a deep-sea pyrene-degrading consortium. Int J Syst Evol Microbiol 61:1370–1374
Langille M, Zaneveld J, Caporaso J, McDonald D, Knights D, Reyes J, Clemente J, Burkepile D, Vega T, Knight R, Beiko R, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31:814–821
Laramée L, Lawrence JR, Greer CW (2000) Molecular analysis and development of 16S rRNA oligonucleotide probes to characterize a diclofop-methyl-degrading biofilm consortium. Can J Microbiol 46:133–142
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Lemarchand K, Berthiaume F, Maynard C, Harel J, Payment P, Bayardelle P, Masson L, Brousseau R (2005) Optimization of microbial DNA extraction and purification from raw wastewater samples for downstream pathogen detection by microarrays. J Microbiol Meth 63:115–126
Long S, Aelion C, Dobbins D, Pfaender F (1995) A comparison of microbial community characteristics among petroleum-contaminated and uncontaminated subsurface soil samples. Microb Ecol 30:297–307
Lundin D, Severin I, Logue JB, Ostman O, Andersson AF, Lindström ES (2012) Which sequencing depth is sufficient to describe patterns in bacterial & α and β-diversity? Environ Microbiol Rep 4:367–372
Luz AP, Pellizari VH, Whyte LG, Greer CW (2004) A survey of indigenous microbial hydrocarbon degradation genes in soils from Antarctica and Brazil. Can J Microbiol 50(5):323–333
MacNaughton SJ, Stephen JR, Venosa AD, Davis GA, Chang Y-J, White DC (1999) Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microb 65:3566–3574
Mangwani N, Shukla S, Kumari S, Rao T, Das S (2014) Characterization of Stenotrophomonas acidaminiphila NCW-702 biofilm for implication in the degradation of polycyclic aromatic hydrocarbons. J Appl Microbiol 117:1012–1024
Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD (2012) PANDAseq: paired-end assembler for illumina sequences. BMC Bioinform 13:31
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618
Mnif I, Mnif S, Sahnoun R, Maktouf S, Ayedi Y, Ellouze-Chaabouni S, Ghribi D (2015) Biodegradation of diesel oil by a novel microbial consortium: comparison between co-inoculation with biosurfactant-producing strain and exogenously added biosurfactants. Environ Sci Pollut R 22:14852–14861
Morais D, Pylro V, Clark IM, Hirsch PR, Tótola MR (2016) Responses of microbial community from tropical pristine coastal soil to crude oil contamination. PeerJ 4:e1733
Morales M, Sentchilo V, Bertelli C, Komljenovic A, Kryuchkova-Mostacci N, Bourdilloud A, Linke B, Goesmann A, Harshman K, Segers F (2016) the genome of the toluene-degrading Pseudomonas veronii strain 1YdBTEX2 and its differential gene expression in contaminated sand. PLoS ONE 11:e0165850
Mukherjee A, Chettri B, Langpoklakpam JS, Basak P, Prasad A, Mukherjee AK, Bhattacharyya M, Singh AK, Chattopadhyay D (2017) Bioinformatic approaches including predictive metagenomic profiling reveal characteristics of bacterial response to petroleum hydrocarbon contamination in diverse environments. Sci Rep 7(1):1108
Nie Y, Chi C-Q, Fang H, Liang J-L, Lu S-L, Lai G-L, Tang Y-Q, Wu X-L (2014) Diverse alkane hydroxylase genes in microorganisms and environments. Sci Rep 4:4968
Oudot J, Merlin F, Pinvidic P (1998) Weathering rates of oil components in a bioremediation experiment in estuarine sediments. Mar Environ Res 45:113–125
Ozaki S, Kishimoto N, Fujita T (2007) Change in the predominant bacteria in a microbial consortium cultured on media containing aromatic and saturated hydrocarbons as the sole carbon source. Microbes Environ 22:128–135
Panicker G, Mojib N, Aislabie J, Bej AK (2010) Detection, expression and quantitation of the biodegradative genes in Antarctic microorganisms using PCR. Anton Leeuw Int J 97:275–287
Parks DH, Tyson GW, Hugenhltz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30:3123–3124
Paulson JN, Pop M, Bravo HC (2011) Metastats: an improved statistical method for analysis of metagenomic data. Genome Biol 12:P17
Peng M, Zi X, Wang Q (2015) Bacterial community diversity of oil-contaminated soils assessed by high throughput sequencing of 16S rRNA genes. Int J Env Res Public Health 12:12002–12015
Połka J, Rebecchi A, Pisacane V, Morelli L, Puglisi E (2015) Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiol 46:342–356
Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35:7188–7196
Rodrigues EM, Morais DK, Pylro VS, Redmile-Gordon M, de Oliveira JA, Roesch LFW, Cesar DE, Tótola MR (2017) Aliphatic hydrocarbon enhances phenanthrene degradation by autochthonous prokaryotic communities from a pristine seawater. Microb Ecol. https://doi.org/10.1007/s00248-017-1078-8
Röling WF, Milner MG, Jones DM, Lee K, Daniel F, Swannell RJ, Head IM (2002) Robust hydrocarbon degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl Environ Microb 68:5537–5548
Santisi S, Cappello S, Catalfamo M, Mancini G, Hassanshahian M, Genovese L, Giuliano L, Yakimov MM (2015) Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium. Braz J Microbiol 46:377–387
Schloss PD (2010) The effects of alignment quality, distance calculation method, sequence filtering, and region on the analysis of 16S rRNA gene-based studies. PLoS Comput Biol 6:e1000844
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. J Appl Environ Microbiol 75:7537–7541
Shao Y, Wang Y, Wu X, Xu X, Kong S, Tong L, Jiang Z, Li B (2015) Biodegradation of PAHs by Acinetobacter isolated from karst groundwater in a coal-mining area. Environ Earth Sci 73:7479–7488
Sorokin DY, Janssen AJ, Muyzer G (2012) Biodegradation potential of halo (alkali) philic prokaryotes. Crit Rev Environ Sci Technol 42:811–856
Sotsky JB, Greer C, Atlas R (1994) Frequency of genes in aromatic and aliphatic hydrocarbon biodegradation pathways within bacterial populations from Alaskan sediments. Can J Microbiol 40:981–985
Suenaga H, Mizuta S, Miyazaki K, Yaoi K (2014) Diversity of extradiol dioxygenases in aromatic-degrading microbial community explored using both culture-dependent and culture-independent approaches. FEMS Microbiol Ecol 90:367–379
Suja F, Rahim F, Taha MR, Hambali N, Razali MR, Khalid A, Hamzah A (2014) Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeter Biodegr 90:115–122
Sun W, Dong Y, Gao P, Fu M, Ta K, Li J (2015) Microbial communities inhabiting oil-contaminated soils from two major oilfields in Northern China: implications for active petroleum-degrading capacity. J Microbiol 53:371–378
Tanase A-M, Ionescu R, Chiciudean I, Vassu T, Stoica I (2013) Characterization of hydrocarbon-degrading bacterial strains isolated from oil-polluted soil. Int Biodeter Biodegr 84:150–154
Van Beilen JB, Funhoff EG (2007) Alkane hydroxylases involved in microbial alkane degradation. Appl Microbiol Biot 74:13–21
Van Beilen J, Li Z, Duetz W, Smits T, Witholt B (2003) Diversity of alkane hydroxylase systems in the environment. Oil Gas Sci Technol 58:427–440
Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN (2015) Synergistic ex situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeter Biodegr 103:116–124
Vasileiadis S, Puglisi E, Arena M, Cappa F, Van Veen JA, Cocconcelli PS, Trevisan M (2013) Soil microbial diversity patterns of a lowland spring environment. FEMS Microbiol Ecol 86:172–184
Vasileiadis S, Puglisi E, Trevisan M, Scheckel KG, Langdon KA, McLaughlin MJ, Lombi E, Donner E (2015) Changes in soil bacterial communities and diversity in response to long-term silver exposure. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiv114
Wang L, Shao Z (2006) Isolation and characterization of 4 benzene/toluene-degrading bacterial strains and detection of related degradation genes. Acta Meteorol Sin 46:753–757
Wang L, Qiao N, Sun F, Shao Z (2008) Isolation, gene detection and solvent tolerance of benzene, toluene and xylene degrading bacteria from nearshore surface water and Pacific Ocean sediment. Extremophiles 12:335–342
Wang X-B, Chi C-Q, Nie Y, Tang Y-Q, Tan Y, Wu G, Wu X-L (2011) Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel Dietzia strain. Bioresource Technol 102:7755–7761
Wang L, Feng L, Zhan Y, Zhu L (2016) Shifts in microbial communitiy structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ Sci Pollut Res 23:14451–14461
Wasi S, Tabrez S, Ahmad M (2013) Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ Monit Assess 185:8147–8155
Wasmund K, Burns KA, Kurtböke DI, Bourne DG (2009) Novel alkane hydroxylase gene (alkB) diversity in sediments associated with hydrocarbon seeps in the Timor Sea, Australia. Appl Environ Microb 75:7391–7398
Wu M, Ye X, Chen K, Li W, Yuan J, Jiang X (2017) Bacterial community shift and hydrocarbon transformation during bioremediation of short-term petroleum-contaminated soil. Environ Pollut 223:657–664
Xue J, Yu Y, Bai Y, Wang L, Wu Y (2015) Marine oil-degrading microorganisms and biodegradation process of petroleum hydrocarbon in marine environments: a review. Curr Microbiol 71:220–228
Yang Y, Wang J, Liao J, Xie S, Huang Y (2014a) Abundance and diversity of soil petroleum hydrocarbon-degrading microbial communities in oil exploring areas. Appl Microbiol Biot 99:1935–1946. https://doi.org/10.1007/s00253-014-6074-z
Yang Y, Wang J, Liao J, Xie S, Huang Y (2014b) Distribution of naphthalene dioxygenase genes in crude oil-contaminated soils. Microb Ecol 68:785–793
Yergeau E, Sanschagrin S, Beaumier D, Greer CW (2012) Metagenomic analysis of the bioremediation of diesel-contaminated Canadian high arctic soils. PLoS ONE 7:e30058
Yuste L, MaE Corbella, MaJ Turiégano, Karlson U, Puyet A, Rojo F (2000) Characterization of bacterial strains able to grow on high molecular mass residues from crude oil processing. FEMS Microbial Ecol 32:69–75
Zrafi-Nouira I, Guermazi S, Chouari R, Safi NM, Pelletier E, Bakhrouf A, Saidane-Mosbahi D, Sghir A (2009) Molecular diversity analysis and bacterial population dynamics of an adapted seawater microbiota during the degradation of Tunisian zarzaitine oil. Biodegradation 20:467–486
Zvyagintseva I, Poglazova M, Gotoeva M, Belyaev S (2001) Effect on the medium salinity on oil degradation by nocardioform bacteria. Microbiology 70:652–656
Acknowledgements
This work was partly financially supported through grants from the Tunisian Ministry of Higher Education and Scientific Research and from the University of Monastir. The authors are grateful to Imed Cheraeif from laboratory of Biochemistry and Mass spectrometry at the Monastir Medical Faculty in Tunisia for his technical assistance and support in the chromatography analysis. We thank Regional Commissariat for the Agricultural Development of Monastir in Tunisia for the technical assistance in physicochemical analyses of soil samples. We are also grateful to Sotirios Vasileiadis for providing a number of R scripts used for bioinformatics analyses and graphs preparation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Gas chromatographic spectra collected after different incubation times (0, 1 and 8 weeks) with Zarzatine crude oil (ZCO). Peaks are labelled according to individual hydrocarbons with their carbon number (C9–C29). (a): chromatograms of control sample (medium with only ZCO); (b) microcosms K; (c) microcosms R; (d) microcosms S; (e) microcosms E. Two replicates per sample are reported. For the control (a), chromatograms at week 0 are reported, while for the inoculated microcosms (b-e) the two graphs above are the replicates at the 1st week, the two below the replicates at the 8th week. SD: External standard. Supplementary material 1 (DOCX 497 kb)
Fig. S2
Percentages of bacterial sequences correctly classified at different taxonomic depth. Data refer to 547,560 sequences identified as bacterial after PCR amplification of the V3-V4 16S rRNA region. Supplementary material 2 (DOCX 70 kb)
Fig. S3
Hierarchical clustering of sequences from all experiments classified at the genus level. Clusters were identified with the average linkage algorithm for taxa that contributed at least 5% to a single sample. Taxa that contributed less than this threshold were added to the sequence group denoted “other”. Supplementary material 3 (DOCX 303 kb)
Rights and permissions
About this article
Cite this article
Omrani, R., Spini, G., Puglisi, E. et al. Modulation of microbial consortia enriched from different polluted environments during petroleum biodegradation. Biodegradation 29, 187–209 (2018). https://doi.org/10.1007/s10532-018-9823-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-018-9823-3