Abstract
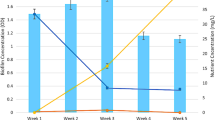
Column experiments were conducted to evaluate the effect of pore velocity on the extent of biodegradation of cis-dichloroethene (cis-DCE) during transport in porous media. Columns were filled with homogeneous glass beads and inoculated with a culture capable of complete dechlorination of tetrachloroethene to ethene. A constant concentration of cis-DCE was maintained in the columns’ influent. Three different pore velocities were tested in duplicate, subjecting each column to a constant velocity. At high flow velocity, degradation of cis-DCE to ethene was nearly complete within the residence time of the columns. However, at medium and low flow velocities, incomplete dechlorination was observed. After 7 weeks, DNA was harvested from the columns to determine differences in the microbial populations. Results suggest that Dehalococcoides sp. were present in higher quantities in the high-velocity columns, consistent with the observed dechlorination. These results suggest that, at contaminated groundwater sites, heterogeneity of groundwater velocity may be one factor that contributes to heterogeneous distribution of biological activity.






Similar content being viewed by others
References
Allen-King RM, Divine PD, Robin MJL, Alldredge JR, Graylord DR (2006) Spatial distributions of perchloroethylene reactive transport parameters in the borden aquifer. Water Resour Res 42 doi:10.1029/2005WR003977
Amos BK, Sung Y, Fletcher KE, Gentry TJ, Wu W-M, Criddle CS, Zhou J, Löffler FE (2007) Detection and quantification of Geobacter lovleyi strain SZ: implications for bioremediation at tetrachloroethene- and uranium-impacted sites. Appl Environ Microbiol 73:6898–6904
Aulenta F, Gossett JM, Papini MP, Rosseti S, Majone M (2005) Comparative study of methanol, butyrate, and hydrogen and electron donors for long-term dechlorination of teatrachloroethene in mixed anaerobic cultures. Biotechnol Bioeng 91:743–753
Behrens S, Azizian MF, McMurdie PJ, Sabalowsky A, Dolan ME, Semprini L, Spormann AM (2008) Monitoring abundance and expression of “Dehalococcoides” species chloroethene-reductive dehalogenases in a tetrachloroethene-dechlorinating flow column. Appl Environ Microbiol 74:5695–5703
Buergmann H, Kleikemper J, Duc L, Bunge M, Schroth MH, Zeyer J (2008) Detection and quantification of Dehalococcoides-related bacteria in a chlorinated ethene-contaminated aquifer undergoing natural attenuation. Bioremediation Journal 12:193–209
Cabirol N, Jacob F, Perrier J, Fouillet B, Chambon P (1998) Interaction between methanogenic and sulfate-reducing microorganisms during dechlorination of a high concentration of tetrachloroethylene. J Gen Appl Microbiol 44:297–301
Carr CS, Hughes JB (1998) Enrichment of high-rate PCE dechlorination and comparative study of lactate, methanol, and hydrogen as electron donors to sustain activity. Environ Sci Technol 32:1817–1824
Cupples AM (2008) Real-time PCR quantification of Dehalococcoides populations: methods and applications. J Microbiol Methods 72:1–11
Cupples AM, Spormann AM, McCarty PL (2003) Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl Environ Microbiol 69:953–959
Cupples AM, Spormann AM, McCarty PL (2004) Vinyl chloride and cis-dichloroethylene dechlorination kinetics and microorganisms growth under substrate limiting conditions. Environ Sci Technol 38:1102–1107
Duhamel M, Edwards EA (2006) Microbial composition of chlorinated ethene-degrading cultures dominated by Dehalococcoides. FEMS Microbiol Ecol 58:538–549
Duhamel M, Edwards EA (2007) Growth and yields of dechlorinators, acetogens, and methanogens during reductive dechlorination of chlorinated ethenes and dihaloelimination of 1, 2-dichloroethane. Environ Sci Technol 41:2303–2310
Duhamel M, Wehr SD, Yu L, Rizvi H, Seepersad S, Dworatzek S, Cox EE, Edwards EA (2002) Comparison of anaerobic dechlorinating enrichment cultures maintained on tetracholoethene, trichloroethene, cis-dichloroethene and vinyl chloride. Water Res 36:4193–4202
Duhamel M, Mo K, Edwards EA (2004) Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl Environ Microbiol 70:5538–5545
Ellis DE, Lutz EJ, Odom JM, Buchanan RJ Jr, Bartlett CL, Lee MD, Harkness MR, DeWeerd KA (2000) Bioaugmentation for accelerated in situ anaerobic bioremediation. Environ Sci Technol 34:2254–2260
Fennell DE, Gossett JM (1998) Modeling the production of and competition for hydrogen in a dechlorinating culture. Environ Sci Technol 32:2450–2460
Fennell DE, Gossett JM, Zinder SH (1997) Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ Sci Technol 31:918–926
Fennell DE, Carroll AB, Gossett JM, Zinder SH (2001) Assessment of indigenous reductive dechlorination potential at a TCE-contaminated site using microcosms, polymerase chain reaction analysis and site data. Environ Sci Technol 35:1830–1839
Fredrickson JK, Garland TR, Hicks RJ, Thomas JM, Li SW, McFadden KM (1989) Lithotrophic and heterotrophic bacteria in deep subsurface sediments and their relation to sediment properties. Geomicrobiol J 7:53–66
He J, Sung Y, Dollhopf ME, Fathepure BZ, Tiedje JM, Löffler FE (2002) Acetate versus hydrogen as direct electron donors to stimulate the microbial reductive dechlorination process at chloroethene-contaminated sites. Environ Sci Technol 36:3945–3952
Hendrickson ER, Payne JA, Young RM, Starr MG, Perry MP, Fahnestock S, Ellis DE, Ebersole RC (2002) Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl Environ Microbiol 68:485–495
Hoelen TP, Cunningham JA, Hopkins GD, Lebrón CA, Reinhard M (2006) Bioremediation of cis-DCE at a sulfidogenic site by amendment with propionate. Ground Water Monit. Remed 26:82–91
Lendvay JM, Löffler FE, Dollhopf M, Aiello MR, Daniels G, Fathepure BZ, Gebhard M, Heine R, Helton R, Shi J, Krajmalnik-Brown R, Major CL, Barcelona MJ, Petrovskis E, Hickey R, Tiedje JM, Adriaens P (2003) Bioreactive barriers: a comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environ Sci Technol 37:1422–1431
Löffler FE, Edwards EA (2006) Harnessing microbial activities for environmental cleanup. Curr Opin Biotechnol 17:274–284
Lookman R, Paulus D, Marnette E, Pijls C, Ryngaert A, Diels L, Volkering F (2007) Ground water transfer initiates complete reductive dechlorination in a PCE-contaminated aquifer. Ground Water Monitor Remed 27:65–74
Lu X, Wilson JT, Kampbell DH (2006) Relationship between geochemical parameters and the occurrence of Dehalococcoides DNA in contaminated aquifers. Water Resour Res 42:W08427. doi:10.1029/2005WR004283
MacDonald JA (2000) Evaluating natural attenuation for groundwater cleanup. Environ Sci Technol 34:346A–353A
Major DW, McMaster ML, Cox EE, Edwards EA, Dworatzek SM, Hendrickson ER, Starr MG, Payne JA, Buonamici LW (2002) Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ Sci Technol 36:5106–5116
Maymó-Gatell X, Chien Y, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568–1571
McCarty PL (1997) Breathing with chlorinated solvents. Science 276:1521–1526
Mendoza-Sanchez I (2007) Effects of pore-scale velocity and pore-scale physical processes on contaminant biodegradation during transport in groundwater: modeling and experiments. PhD Dissertation, Texas A & M University, College Station, TX
Muyzer G, DeWaal EC, Uitierlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Env Microbiol 59:695–700
Ritalahti KM, Amos BK, Sung Y, Wu Q, Koenigsberg SS, Löffler FE (2006) Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl Environ Microbiol 72:2765–2774
Rittmann BE (1993) The significance of biofilms in porous media. Water Resour Res 29:2195–2202
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill, New York
Sandrin SK, Brusseau ML, Piatt JJ, Bodour AA, Blanford WJ, Nelson NT (2004) Spatial variability of in situ microbial activity: biotracer tests. Ground Water 42:374–383
Schaefer CE, Condee CW, Vainberg S, Steffan RJ (2009) Bioaugmentation for chlorinated ethenes using Dehalococcoides sp.: comparison between batch and column experiments. Chemosphere 75:141–148
Scheutz C, Durant ND, Dennis P, Heisterberg-Hansen M, Jørgensen T, Jakobsen R, Cox EE, Bjerg PL (2008) Concurrent ethene generation and growth of Dehalococcoides containing vinyl chloride reductive dehalogenase genes during an enhanced reductive dechlorination field demonstration. Environ Sci Technol 42:9302–9309
Sinclair JL, Randtke SJ, Denne JE, Hathaway LR, Ghiorse WC (1990) Survey of microbial populations in buried-valley aquifer sediments from northeastern Kansas. Ground Water 28:369–377
Sleep BE, Seepersad DJ, Mo K, Heidorn CM, Hrapovic L, Morrill PL, McMaster ML, Hood ED, LeBrón C, Sherwood-Lollar B, Major DW, Edwards EA (2006) Biological enhancement of tetrachloroethene dissolution and associated microbial community changes. Environ Sci Technol 40:3623–3633
Smits THM, Devenoges C, Szynalski K, Maillard J, Holliger C (2004) Development of a real-time PCR method for quanitification of the three genera Dehalobacter, Dehalococcoides, and Desulfitobacterium in microbial communities. J Microbiol Methods 57:369–378
Tas N, van Eekert MHA, Schraa G, Zhou JZ, de Vos WM, Smidt H (2009) Tracking functional guilds: “Dehalococcoides” spp. in European river basins contaminated with hexachlorobenzene. Appl Environ Microbiol 75:4696–4704
Waller AS, Krajmalnik-Brown R, Löffler FE, Edwards EA (2005) Multiple reductive-dehalogenase-homologous genes are simultaneously transcribed during dechlorination by Dehalococcoides-containing cultures. Appl Environ Microbiol 71:8257–8264
Yang Y, McCarty PL (1998) Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ Sci Technol 32:3591–3597
Acknowledgments
The authors appreciate the help of: Dr. Chang Ping for laboratory supervision in the DNA extraction; SiREM for providing the KB-1® culture used in this research; and Sandra Dworatzek, Phil Dennis, and Elizabeth Ney (all of SiREM) for their technical advice and assistance in the DGGE and qPCR analysis. The authors thank an anonymous reviewer for a thorough and constructive review of a previous version of this paper. Financial support for this research has been provided to Thomas J. McDonald from the National Institute of Environmental Health Sciences (NIEHS) through the Superfund Grant #P42 ES04917, and to Itza Mendoza from the following sources: Consejo Nacional de Ciencia y Tecnología (CONACYT), México; Instituto Politécnico Nacional (IPN), Ciudad de México, México; and the United States Geological Survey (USGS) through the Texas Water Resources Institute (TWRI). Any opinions, findings, conclusions, or recommendations expressed in this paper are those of the authors and do not necessarily reflect the views of NIEHS, CONACYT, IPN, USGS, or TWRI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendoza-Sanchez, I., Autenrieth, R.L., McDonald, T.J. et al. Effect of pore velocity on biodegradation of cis-dichloroethene (DCE) in column experiments. Biodegradation 21, 365–377 (2010). https://doi.org/10.1007/s10532-009-9307-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-009-9307-6