Abstract
Microplastic (MP) occurrence in wastewater poses serious threats to aquatic organisms due to their ecological impact. Additionally, these microplastics may provide a unique habitat for microbial biofilms. This study explored the possible factors that facilitate biofilm formation on different MPs in a wastewater environment. Biofilm formation was determined via measurement of optical density (OD) and confirmed using scanning electron microscopy. Furthermore, microbial community profiling was performed via 16S rRNA amplicon sequencing. The highest biofilm formation occurred after 3 weeks of exposure, followed by a decline to its lowest after 5 weeks. Biofilms were predominant on MPs that were exposed to dark conditions, mesophilic temperature (25 °C) and aerobic conditions. Under these conditions, a positive correlation was observed between the OD value and ammonia (NH3) (r = 0.824) and nitrite (NO2) concentrations (r = 0.1). However, a negative correlation (r = −0.673) was found between the OD value and nitrate (NO3) levels in the medium. Furthermore, the highest biofilm formation was observed on polyethylene particles. The most dominant phyla in both the biofilms and wastewater medium were Proteobacteria, Bacteroidetes and Planctomycetes, with Methylotenera being the most abundant genera in the biofilms. In general, the polypropylene particles supported the most diverse bacterial community (H' = 2.51138 and Simpson index = 11.096), while high-density polyethylene supported the least diverse bacterial community (H' = 0.88779 and Simpson index = 1.5324). The study also demonstrated that both UV and chlorine treatments were effective in inactivating these biofilms, within 30 and 10 min, respectively.
Similar content being viewed by others
Introduction
The aquatic environment is becoming increasingly polluted with plastic debris. The production and widespread use of plastic materials has led to a significant increase in pollution rates (Okshevsky et al. 2020). These pollutants pose numerous hazards to aquatic ecosystems, including the risks of direct ingestion by aquatic animals, transfer and bioaccumulation within the food web, as well as the transmission of harmful heavy metals, organic pollutants and pathogenic microbes (Chapron et al. 2018). The greatest challenge associated with plastic occurrence in the aquatic environment is posed by microplastics (MPs). These MPs are susceptible to colonization by microorganisms when they come into contact with water, resulting in the formation of biofilms (Kelly et al. 2021). The development of a biofilm generally involves a complex three-phase process, consisting of attachment, maturation and detachment stages (Saxena et al. 2019). Factors such as temperature, light/dark conditions, oxygen levels and nutrient availability can influence the process of biofilm formation (Akoğlu 2020).
The hydrophobicity and texture of attaching surfaces are also known to contribute to biofilm formation (Shen et al. 2019; Okshevsky et al. 2020). For instance, there may be a greater tendency for microorganisms to colonize particles with textured/rough surfaces as compared to particles with smooth surfaces (Shen et al. 2019). Wastewater treatment plants (WWTPs) are one of the major routes for MPs to enter the environment, and they also provide an ideal environment for the formation of biofilms. MPs are exposed to various physical and chemical factors in wastewater that may influence the development of biofilms (Tallawi et al. 2017; Sajjad et al. 2020; Li et al. 2022).
Biofilm-forming bacteria have certain physiological features, including flagella, fimbriae and pili, that are helpful in the process of biofilm formation (Flemming et al. 2017). Both Gram-negative and Gram-positive bacteria can form biofilms, but the most common forms reported from MP surfaces are Pseudomonadaceae, Moraxellaceae, Enterobacteriaceae and Comamonadaceae (Khatoon et al. 2018; Kelly et al. 2021). These microorganisms have been reported to be unique compared to the microbial community in the water environment and are therefore termed “plastispheres” (Amaral-Zettler et al. 2020). Recent findings indicate that microorganisms attached to MPs can be transported and dispersed over significant distances (Bowley et al. 2021). Additionally, MP surfaces with biofilm could potentially promote horizontal gene transfer (HGT) between bacteria, which could lead to the transfer of antibiotic-resistant and pathogenic genes between bacterial communities (Karkman et al. 2017).
Therefore, MPs may play a role in the transport of pathogens and antibiotic-resistant genes (ARG) in the aquatic environment (Hoellein et al. 2017), especially from WWTPs. This is because microorganisms inside the biofilm are protected from surface detachment, predation, inhibitory and degrading mechanisms (Rittmann 2018). In this way, biofilms may protect microorganisms and MPs from tertiary treatment methods such as ultraviolet (UV) irradiation and chlorination during wastewater treatment, reducing the efficiency of these processes (Sun et al. 2019; Liu et al. 2016; Rittmann 2018).
Despite the potential impact of MP occurrence in WWTPs, limited knowledge exists regarding the factors that facilitate biofilm formation on MPs, the microbial community associated with these MPs and the effects of tertiary wastewater treatment. This study focuses on identifying those factors that facilitate biofilm formation. The bacterial population was also investigated to determine the microbial composition of biofilms attached to three different types of MPs and the wastewater medium during a five-week incubation under controlled conditions. Additionally, the impacts of chlorine and UV tertiary wastewater treatment processes were determined to investigate the effects of common disinfection methods on MP-associated biofilms during wastewater treatment.
Materials and methods
Microplastic particles
Three types of MPs, namely high-density polyethylene (HDPE), low-density polyethylene (LDPE) and polypropylene (PP), with a diameter of 5 mm, were acquired from Merck (PTY) LTD, South Africa, for use in this study.
Biofilm formation experiment
In this experiment, exposure time, temperature, light/dark conditions and aeration/non-aeration were chosen to determine their effects on MP biofilm formation. Untreated wastewater was collected from a domestic wastewater treatment plant in Durban for the experiment. The wastewater was then filtered using a mesh sieve (100 µm pore size) to remove larger suspended particles. MPs (2 g each) of different types were added to each Erlenmeyer flask filled with 100 mL of filtered wastewater. Subsequently, the flasks were exposed to various combinations of conditions as outlined in Table 1. Each experiment had a maximum exposure time of five weeks to determine the optimal duration for biofilm formation on the MPs. To investigate the influence of temperature, separate experiments (Experiments 1 to 8, Table 1) were conducted at three different incubation temperatures: 20 °C, 25 °C and 35 °C. The dark conditions were achieved by covering the flasks with foil and preventing light from entering, keeping all other parameters similar to those of the light conditions. Aerobic conditions were achieved by covering the flasks with perforated gauze. Anaerobic conditions were generated in sealed reactors that were initially purged with nitrogen gas. All types of MPs used in this study, including both rough and smooth MPs, were subjected to the aforementioned conditions.
Analysis of biofilm formation using optical density measurements
Optical density (OD) measurements were used to assess biofilm formation on MPs. This was achieved by collecting MPs (10) from each experimental flask at weekly intervals, washing them with sterile water and inoculating them on nutrient broth (10 mL). Following 24 h of incubation, the flasks containing MPs were vigorously shaken using a vortex for 1 min to detach the biofilms. The OD was then measured at 660 nm using a Spectroquant pharo300 Spectrophotometer.
Nutrient analysis
Wastewater samples (5 mL) were collected from the flasks (used above in Sect. "Biofilm formation experiment") during week 1, week 3 and week 5 for nutrient content analysis. The samples were filtered through 0.45-μm syringe filters before the analysis. Nitrate (NO3−), nitrite (NO2−) and ammonia (NH4) concentration in the wastewater was measured using the Gallery discrete Autoanalyzer (Thermo Fisher Scientific, USA).
Scanning electron microscopy
A scanning electron microscope (SEM) analysis was conducted to confirm the attachment of bacteria to MP surfaces after exposure to a wastewater medium. Recovered MPs were fixed in 2.5% glutaraldehyde for 8 h at 4 °C. After fixation, samples were rinsed with 0.1 M sodium phosphate buffer (pH 7.2) and dehydrated with alcohol series consisting of 30%, 50%,70%, 90%, 95% and 100% alcohol (10 min each). To minimize distortion of the microplastics before SEM analysis, critical point drying (CPD) was determined. The particles were then mounted onto a stub, covered in carbon glue and coated with gold palladium. The stub was then placed in the SEM, and images were recorded and stored.
Profiling of microbial community attached to microplastics and in wastewater medium
Experimental setup
The favorable conditions for biofilm formation determined and described in Sect. "Biofilm formation experiment" were used for the following experiment. The conditions followed included: an incubation temperature of 25 °C, under dark and aerobic conditions. Sampling for microbial analysis was done during week 1, week 3 and week 5.
Sample preparation
Two ml wastewater samples were was in Eppendorf tubes and centrifuged at 2795 × g for 5 min. The supernatant was discarded, and the pellet was resuspended in 1 mL distilled water and centrifuged at 2795 × g for another 5 min. Thereafter the pellet was resuspended in 1 mL PBS and centrifuged again at 2795 × g for 5 min. DNA was extracted from the resulting pellet. MPs (10 particles) were removed from the flasks, rinsed twice with sterile distilled water and collected in Eppendorf tubes for direct DNA extraction.
DNA extraction, sequencing and analysis
Total DNA was extracted from both wastewater and MPs using the phenol/chloroform extraction method adapted from Awolusi et al. (2018). The purity and quantity of the DNA extracts were analyzed by spectrophotometry using the IMPLEN NanoPhotometer. The DNA extracts were then sent for 16S rRNA amplicon sequencing and analysis at Inqaba Biotec (Pretoria, South Africa).
Data analysis
The fastq files from 16s rRNA sequencing were subjected to a DADA2 and a QIIME 2 pipeline. Alpha diversity was applied in analyzing the complexity of species diversity for a sample through 20 different indices, including observed species, Shannon and Simpson. All these indices in the samples were calculated with QIIME. Beta diversity and principal coordinate analysis (PCoA) were calculated based on the similarity and distance between the samples. This includes the non-phylogenetic Bray–Curtis distance and phylogenetic-based weighted UniFrac distance. The outputs of the DADA2 pipeline were further subjected to an R studio pipeline to quantify the phylogenetic relationship and abundance of species in the sample.
Tertiary treatment
Chlorine treatment
Flasks containing MPs in a wastewater medium after week 5 of incubation were exposed to 12.5% sodium hypochlorite with a concentration of 5 mg/L for 10, 20, 30 and 60 min (WWTP conditions) (USEPA 1999). The treated MPs were collected at each interval for further analysis.
UV treatment
MPs from flasks containing a wastewater medium after week 5 of incubation were transferred into petri dishes. The petri dishes were illuminated with ultraviolet light emitting 254 nm at a distance of 10 cm from the UV-C source (Lin et al. 2020). Exposure was carried out for 10, 15, 30 and 60 min. Samples of MPs were collected at each interval for further analysis.
Heterotrophic plate count (HPC)
To ascertain the effects of UV radiation and chlorine on biofilms, HPC was performed as per standard methods for water and wastewater testing (APHA 2011). Approximately 10 MPs were collected from flasks before treatment (control) and thereafter from each treatment and placed in an Eppendorf tube containing 2 mL of phosphate-buffered saline (PBS). The tubes were then vigorously shaken and vortexed for one minute to achieve the removal of the biofilms attached to the MPs. Thereafter, about 0.2 mL of biofilm suspension was spread over m-HPC agar and incubated for 48 h at 35 °C. CFU/MP was determined by counting colonies and by using the following equation:
Statistical analysis
The data were captured in Excel (Microsoft Corp., USA), and diamond plots were used to represent the distribution of the data obtained for biofilm concentrations under the various experimental conditions. Each box plot illustrates the estimated median (center line), upper and lower quartiles (box limits), interquartile range (whiskers) and outliers (points). The median biofilm concentration values were compared to determine the concentration of biofilms formed on HDPE, LDPE and PP. To determine the significance between light and dark conditions, aerobic and anaerobic conditions, and rough and smooth microplastics, the t-test was applied. The analysis of variance (ANOVA) was used to demonstrate the significance of temperature (20 °C, 25 °C and 35 °C) and MP types (HDPE, LDPE and PP) on biofilm formation, and Pearson correlation coefficient (r) was used to determine the relationship between biofilm concentration and nutrient concentration in the media.
Results and discussion
Relationship between biofilm formation, exposure time and nutrient concentrations in the wastewater
The formation of biofilm was evident in all experiments regardless of the conditions. The biofilm formation was quantified based on the OD value. The highest amount of biofilm formation was observed at week 3 (OD = 1.77), followed by a subsequent decline in the OD value. By week 5, the OD value reached its lowest point (OD = 1.1), indicating a decrease or detachment of the biofilm after a certain duration. Furthermore, the SEM images presented in Figs. A1 and A2 (in Appendix A) provided visual evidence of biofilm formation on the MPs.
A comparative analysis of the nutrient concentrations in the wastewater revealed a decrease in ammonia (NH4) concentrations from 33,45 mg/L to 7,95 mg/L from week 1 to week 5 while nitrate (NO3) concentrations increased from 0 mg/L to 40,65 mg/L. The concentration of nitrite (NO2) increased from 0.01 mg/L in week 1 to 0.74 mg/L in week 3 and then decreased to 0 mg/L in week 5 (Fig. 1). The correlation analysis revealed a positive linear relationship between biofilm concentration and NO2 (r = 0.824) and NH4 (r = 0.1). In contrast, the correlation analysis for biofilm concentration and NO3 revealed a negative linear relationship (r = −0.673). However, statistically insignificant correlations were observed between biofilm concentration and NO3 (P-value = 0.265), NH4 (P-value = 0.532) and NO2 (P-value = 0.808).
Impact of selected factors on biofilm formation
Impact of factors on biofilm formation on PP
The combined conditions that led to the highest biofilm formation on PP differed as the weeks progressed. During the first week, the D–A–R (dark, aerobic and rough MPs) setup showed the highest median biofilm concentration of 1.8, while the L–A–S demonstrated the lowest. Likewise, the highest median concentration, of 1.2, was observed on the D–AN–S experimental setup at week 4, whereas by week 5, the highest concentration was observed on the L–A–R experimental setup (Fig. 2A).
The biofilm formation under the three different temperature conditions varied significantly compared to the concentrations over time. At 20 °C, the L–A–R had the highest median concentration (1.7). At 25 °C and 35 °C, the highest median concentrations were observed in the D–A–R conditions (Fig. 2B). Despite these differences, the results further indicated that rough MPs under aerobic conditions at 35 °C facilitated the formation of higher biofilms.
Impact of factors on biofilm formation on HDPE
With respect to HDPE, in the first week of the study, the D–A–S had a median biofilm concentration of 1.6, while the lowest biofilm concentration was observed in the D–A–R. At weeks 4 and 5, the highest median concentrations (1.2 and 1.1) were recorded on the L–A–R. The biofilm concentration on HDPE also differed under the three different temperatures. At 20 °C, the highest median concentration was found on the D–AN–S. On the other hand, at 25 °C, the highest median concentration (1.8) was observed for L–A–R while at 35 °C, the highest concentration was observed on the D–AN–R experimental setup (Fig. 3B). Overall, the highest median biofilm concentration was observed in L–A–R at 25 °C.
Impact of factors on biofilm formation on LDPE
In this experimental setup, during week 1, the D–A–R produced the highest median biofilm concentration (1.7), while the L–AN–R generated the lowest median biofilm concentration (1.3). In general, the combination of conditions that had the greatest impact on biofilm formation varied over time. Figure 4A shows a similar pattern with PP (Fig. 2A) and HDPE (Fig. 3A) as well as a decrease in biofilm concentration after week 3. By week 4, the highest median concentration (1.4) was observed on the D–A–R, and by week five, the highest median concentration (1.3) was detected on the L–AN–S experimental setup. The biofilm concentrations at the three individual temperature conditions varied, but the combined conditions with the highest impact on biofilm formation were similar at both temperatures. Specifically, at 20 °C and 35 °C, the highest median biofilm concentrations of 1.6 and 1.8 were observed for D–AN–R (Fig. 4B). However, at 25 °C, the highest median biofilm concentration of 1.8 was found on the L–A–R. Despite the differences, results indicated that D–AN–R at 35 °C facilitated the most biofilm formation.
Bacterial diversity in biofilms and wastewater medium
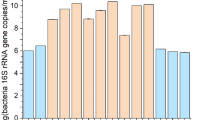
The profile of the microbial community from both biofilms and the surrounding wastewater medium was determined via 16S rRNA-based metagenomic sequencing. A comparison of OTUs for wastewater medium samples across the weeks (week 0 to week 5) indicated a variation in species richness between week 0 (WW initial) and week 5 (WW5) (Table 2). The lowest species richness (1 OTU) was observed during week 0, while the highest species richness (6 OTUs) was observed during week 1, and by week 5, the species richness had decreased to 4 OTUs.
A similar trend was observed for biofilms samples. The species richness of biofilm from LDPE was the highest (17 OTUs) at week 1, and the lowest (4 OTUs) was observed at week 5, whereas for PP the highest (18 OTUs), which was also the overall highest species richness, was observed during week 3. During week 5, PP biofilms also had the lowest species richness (5 OTUs). The Shannon indices of the wastewater medium ranged from 0 to 4 from week 0 to week 5. For HDPE, LDPE and PP, it ranged from 0.88779 to 1.46499, 1.24754 to 2.292 and 1.24963 to 2.51138, respectively. The highest species diversity (H’ = 2.51138) in biofilms was observed for PP during week 1, while the lowest species diversity (H’ = 0.88779) was observed for HDPE during week 3. The Simpson indices of the wastewater medium ranged from 1 to 4.18103 from week 0 to week 5. For HDPE, LDPE and PP, it ranged from 1.5324 to 2.54267, 3.1544 to 7.33374 and 2.74015 to 11.096, respectively. The highest species diversity (Simpson index = 11.096) in biofilms was observed for PP during week 1 while the lowest species diversity (Simpson index = 1.5324) was observed for HDPE during week 3. It was found that PP supported the most diverse (H’ = 2.51138 and Simpson index = 11.096), while HDPE supported the least diverse species (H’ = 0.88779 and Simpson index = 1.5324). The second and third principle coordinates account for 13.72% and 12.49% of the variation of the data set. On this principle, similarities are elucidated based on “dissimilarities.” The results on PCoA in Fig. 5 suggest that there may be dissimilarities in OTUs in HDPE, LDPE and control samples. PP and LDPE samples may share the most features because the randomly selected representative OTUs were found to be similar in abundance in LDPE and PP samples.
Bacterial abundance in biofilms and wastewater medium
The most dominant phyla in the biofilms and wastewater medium were Proteobacteria, Bacteroidetes and Planctomycetes (Fig. 6). The abundance of Proteobacteria remained the highest throughout the study period. During week 5, biofilms had the highest abundance of Proteobacteria at 91.28%, and the lowest abundance of Proteobacteria (73.55%) was observed during week 1. The abundance of Bacteroidetes in biofilms decreased from week 1 to week 5. The highest abundance of Bacteroidetes of 15.18% was observed on week 1 and thereafter decreased to 0.875% by week 5. The abundance of Planctomycetes increased from week 1 to week 3 and thereafter decreased by week 5. The highest abundance of Planctomycetes of 8.73% in biofilm samples was observed during week 3. The lowest abundance of Planctomycetes of 2.73% was observed during week 5.
The bacterial abundance associated with the different types of microplastics
MPs exhibited different bacterial populations in biofilms. It was observed that Methylotenera was the most abundant genus on HDPE during week 1 (34.16%) and week 3 (42.45%). For LDPE, the most abundant genus during week 1 was Hydrogenophaga with an abundance of 19.07%; however, during weeks 3 and 5 the most abundant genus was Methylotenera (25.39%) and Rhodanobacter (14.76%), respectively. The most abundant genus observed on PP during week 1 was Nevskia (12.05%), and during weeks 3 and 5, the most abundant genus was Methylotenera (36.85%) and Rhodanobacter (32.61%), respectively. Interestingly, it was observed that during week 3, the most abundant genus was Methylotenera for the three different types of MPs.
The difference in bacterial abundance in the biofilm and wastewater
A variation was observed between the bacterial population in the biofilms and the wastewater medium (Fig. 7). For instance, during week 1, the most abundant genus present on the microplastics were Methylotenera, Hydrogenophaga, Nevskia and Zooglea, whereas the most abundant genus in the wastewater during the same period was C39 (45.25%). Additional genera such as Planctomycetes, Sediminibacterium Polynucleobacter and Prosthecobacter were also found in wastewater during week 1. Methylibium and Rhodanobacter were the two genera that were present in both biofilms and wastewater medium during week 5. The most abundant genus in wastewater was Luteimonas (18.96%) which was not present in biofilm. Additional genera such as Gemmata, Nitrospira and Methylibium were found in the wastewater medium and not in biofilms. In general, LDPE biofilm samples had the largest abundance at a class level and shared 49 of the 661 read counts with PP biofilm samples (Fig. 7). The sampling bias against HDPE samples indicates that it may have had the largest occupation of read counts than the other sample groups, but is unclear due to sampling at 1 week and 3 weeks.
Impact of chlorine and UV on microplastic-bound microorganisms and the wastewater
Inactivation of attached biofilms was found to be effective with both UV and chlorine treatment. According to the results (Table 3), the initial biofilm on MPs contained 15000 CFU/MP. Following 10 min of UV treatment, a significant reduction was observed, with only 2.5 CFU/MP remaining. During the subsequent 30 min of treatment, colony growth was still observed, but at a significantly reduced level (1 CFU/MP). However, beyond this period, no colony growth was observed. Similarly, there was no evidence of colony growth following chlorine treatment (5 mg/L) for 10 to 60 min (Table 3), indicating that chlorine treatment (optimum level of 5 mg/L) for up to 10 min is effective in inactivating microbes attached to the microplastics. The biofilm inactivation percentage reached 100% after 60 min of UV treatment, whereas 100% inactivation was achieved with chlorine treatment after only 10 min of treatment.
Discussion
A comparison of biofilm formation under different wastewater conditions
Microorganisms have a remarkable ability to colonize MPs in aquatic environments. When MPs are exposed to water, such as wastewater, for a prolonged period, they become covered with biofilms consisting of microorganisms (Amaral-Zettler 2020; Kirstein et al. 2018). For instance, in the current investigation, the connection of microbes was discovered when microplastics were exposed to wastewater for a week. Microbial interaction is first established by both non-selective and selective adhesion processes (Huang et al. 2019). Extracellular polymeric substances (EPS), which are prone to be secreted by microbes once they adhere to a surface, aid in the adhesion of microorganisms (Wang et al. 2021a, b). Sand abrasion, water shear stress and photodegradation are a few examples of the physical and chemical stresses that EPS can shield microorganisms against. In a biofilm, EPS can be either soluble or bound. Sheaths, weakly bound polymers and capsular polymers are all examples of bound EPS, while colloids, slimes and soluble macromolecules are soluble EPS (Tu et al. 2020).
Soluble and bound EPS has been observed on the surface of microplastics with biofilms in laboratory studies. For example, Tu et al. (2020) investigated the formation of biofilms on PE film and discovered that after 135 days, the biofilm had released a substantial amount of extracellular polysaccharides. The current investigation found that 3 weeks of exposure resulted in the strongest microbial association based on the OD measure. This is equivalent to about 21 days of exposure, which indicates a shorter time for maximum attachment compared to the results obtained by Tu et al. (2020). This may be due to several factors, including the availability of nutrients and the microbial community in the medium. For instance, Webb et al. (As referenced in Wang et al. 2021a, b) proposed that the carbon/nitrogen ratio was connected to the creation of EPS. MPs become sticky when exposed to EPS, which encourages the development of heteroaggregates of MPs, microbes and chemicals (Rummel et al. 2017). Microorganism growth leads to the maturation of biofilm. At this stage, biofilm transitions from a 2-D to a 3-D structure have a complex architecture and contain immobilized bacteria (Kataky and Knowles 2018). Then, bacteria have a propensity to break out from the biofilm and cling to a fresh surface. The results of the OD measurements indicated a decrease in microbial association during the fourth and fifth weeks, suggesting that biofilm dispersion commenced in the current study from week 4. The environmental parameters (such as light intensity, temperature and nutrients) as well as the characteristics of the microplastics have an impact on the development of biofilm on them (e.g., type and roughness). Nitrogen and phosphorus, for example, have an impact on the metabolism of carbon in the biofilm on microplastics (Miao et al. 2021). The growth rate of biofilms on microplastics is positively correlated with total phosphorus and total nitrogen (Li et al. 2018). These nutrient-rich settings stimulate bacteria to change from a planktonic state to a biofilm form, whereas nutritional deficiency causes biofilms to separate from surfaces (Sehar and Naz 2016). In this study, a positive correlation was found (r = 0.677) between the concentration of biofilms and the concentration of nitrite and ammonia in the media under aerobic conditions. In contrast, the biofilm concentration exhibited a negative correlation with nitrate under similar conditions. It may be that the decline in biofilm formation in week 5 was due to a decrease in nutrient concentration, which resulted in the aging of the biofilm and subsequent detachment from the MPs (Huang et al. 2019; Toyofuku et al. 2016). Furthermore, it is noteworthy that under aerobic conditions, the conversion of ammonia to nitrite and nitrate was observed at various stages of biofilm development, suggesting the presence of nitrifying bacteria in the microbial community within the biofilm. The accumulation of nitrate under aerobic conditions may suggest that anaerobic denitrifiers are not active in these conditions. This suggests that the composition of wastewater and operational/environmental conditions may influence the structure of microbial communities within biofilms (Liu et al. 2016). Biofilm formation may be influenced by temperature as the enzyme reaction rate is directly affected by temperature. Additionally, studies have shown that bacteria have a greater surface area at low temperatures than at higher temperatures (Govaert et al. 2018). In a study conducted by Townsley and Yildiz (2015), biofilms formed by Vibrio cholerae at 15 °C and 25 °C demonstrated greater thickness and better structure than biofilms formed at 37 °C. Studies have also linked the temperature effect with the number of bacterial appendages. For example, when the number of flagella increases, the surface area of bacteria increases, and the opportunity for bacterial adhesion increases (Townsley and Yildiz 2015). In this study, the findings corroborated those in the previous literature since the highest median biofilm values were observed at 25 °C.
The development of biofilm is greatly influenced by the characteristics of MPs. The microorganism populations in biofilms are directly influenced by the types of MPs. Stronger hydrophilicity in microplastics results in greater affinity for bacteria (Pinto et al. 2019). While LDPE and HDPE differ chemically depending on how many PE chains are compressed into the polymer chains, the surfaces of PP are more hydrophobic than PE (Hossain et al. 2019). The difference in the quantity of biofilm on the various types of MPs in the current investigation was not statistically significant. This suggests that while the kind of polymer may not have had a major influence on the amount of biofilm adhered to the MPs, it may have had a substantial impact on the biofilms' composition (Yang et al. 2020). Furthermore, it was observed in the current study that the amount of biofilms on the rough MPs was higher compared to the smooth MPs. The findings of the study might be explained by the fact that microorganisms are more likely to adhere to microplastics with rough surfaces (Nauendorf et al. 2016; Miao et al. 2021; Feng et al. 2020; Parrish and Fahrenfeld 2019). Microorganisms can cling to MPs in gaps that are produced, and rough surfaces promote nutrient absorption, which facilitates microbe adhesion (Wang et al. 2021a, b). Availability of oxygen and light was also determined to impact the formation of biofilms or the association of microorganisms with MPs. Biofilms are shaped by the interactions between autotrophic and heterotrophic microbes (Schmidt et al. 2018). This study observed that biofilm formation occurred at a greater rate under dark conditions as compared to light conditions. The prevalence of slow-growing autotrophs is high in ambient light conditions since light is the primary source of energy for these organisms (Schmidt et al. 2018), while they are low in dark or dim light conditions (Pinto et al. 2019). It is likely that, under dark conditions, more rapidly growing heterotrophic bacteria grow in the biofilms, which could explain the results obtained in the current study. Earlier studies suggested that low oxygen levels enhance biofilm formation while normal oxygen levels may decrease biofilm formation (Totani et al. 2017). The present study, in contrast, has shown a significantly higher biofilm formation rate under aerobic conditions than under anaerobic conditions.
Microbial profile of biofilms associated with MPs
There has been growing consensus in recent years that bacterial populations in plastispheres are markedly different from those that are present naturally in aquatic bodies (De Tender et al. 2015).
In comparison with water habitats, there is less research that has assessed bacterial OTUs in the plastisphere and surrounding water in wastewater settings. In the current study, the bacterial communities on MPs were more diverse and richer than those in the wastewater medium (Table 2). The more diverse biofilm communities compared to the aqueous medium may be evidence of the bacteria' substrate selectivity on MPs during biofilm growth (Wen et al. 2020). Similar results have also been found in several investigations. For instance, in contrast to free-living bacterial communities from a sewage pipe in Beijing, China, the bacterial communities from the incubated MPs exhibited higher OTU counts (Zhao et al. 2021). On the other hand, the plastisphere displayed lower OTU counts than the surrounding effluent from a sewage pipe in Beijing, China, when compared to it (Wang et al. 2021b). Additionally, compared to unincubated plastic, incubated plastic in synthetic wastewater from WWTPs in New Jersey, USA, had a greater bacterial community abundance (Pham et al. 2021). The neighboring water from the German WWTP contained a larger percentage of OTUs, whereas the deployed MPs had a lower percentage (Oberbeckmann et al. 2017). In comparison with the nearby water, MPs cultivated in an Italian WWTP showed a reduced predicted OTU richness (Eckert et al. 2018).
Bacteria belonging to Proteobacteria were the most dominant in the biofilms, followed by Bacteroidetes and Planctomycetes (Fig. 6). According to Zhang et al (2019), Proteobacteria are reported as one of the most dominant biofilm-forming phyla. A previous study done by Wu et al. (2019) reported that Proteobacteria and Planctomycetes as the most abundant phyla found on MPs and they were much higher in abundance in MP biofilms than on other natural substrates. Wu et al (2019) also found that the abundance of Bacteroidetes in MP biofilms was lower than in biofilms on other natural substrates; however, in this study, Bacteroidetes was a dominant phylum in the biofilms. However, it must be noted that microbial communities on other substrates, other than MPs, were not considered in the current study; therefore, the community difference between MPs and other substrates cannot be compared in the current study. During the five weeks, the abundance of Bacteroidetes and Planctomycetes decreased from week 1 to week 5, whereas the abundance of Proteobacteria increased from week 1 to week 5 and then remained the highest throughout the study period. A study done by Miao et al. (2019) reported that in a 3-week culture study, Proteobacteria remained dominant in all biofilm samples followed by Bacteroidetes, indicating that irrespective of time Proteobacteria was the dominant phyla in biofilms.
The kind of polymer has a considerable impact on the makeup of the MP bacterial population, according to earlier investigations. For instance, Frère et al (2018) found that the bacterial community of polystyrene (PS) was unique from that of PE and PP. Bacillus species and Shewanella, Bdellovibrio and Lewinellafor, Faecalibacterium and Veillonella, and Pseudoalteromonas and Alteromonas, respectively, were shown to be the marker taxa of polyvinyl chloride (PVC), PP, PE and PS by Li et al (2018). According to Feng et al (2020), Pseudomonas may grow on PS, polyethylene terephthalate (PET) and LDPE. Another study by Hansen et al (2021) compared the microbial communities on PE, PP and PS plastics and reported a significant difference in the biofilms. The most abundant genera found on the three types of MPs, in the current study, were Methylotenera, Zooglea, Hydrogenophaga, Rhodanobacter and Nevskia. The abundance and presence of these genera differed among the three types of MPs, but Methylotenera remained dominant. A study done by Rummel et al (2021) reported Methylotenera as the dominant primary colonizing genus. Methylotenera sp. are known as biofilm-forming bacteria that can secrete high amounts of EPS (Chen et al. 2022). In this study, Luteimonas, Nitrospira, Gemmata and C39 are the genera that were found in the wastewater and not in biofilms. The Nitrospira found in wastewater during week 5 could be ammonium oxidizing Nitrospira (comammox). Previous studies have found comammox Nitrospira able to oxidize both ammonia and nitrite in a single cell (Takahashi et al. 2020; van Kessel et al. 2015). This could be the reason for the nitrate production that was observed in the current study.
The impact of UV and Chlorine tertiary treatment on biofilms
MPs have been detected in wastewater treatment plant effluent, and they have been demonstrated to serve as habitats and transport vehicles for microbial pathogens. This highlights the importance of tertiary treatment in the process of inactivating attached microbes (Kaur and Roy 2021). Biofilms resist tertiary treatment primarily by forming thick matrices, and UV radiation can only penetrate the top layer of microbial cells (de Carvalho 2017). UV-C radiation reacts with DNA and RNA hindering replication and transcription processes and therefore inactivates the growth of microorganisms (Rosario et al. 2021). It has been suggested that the effectiveness of UV radiation treatment is also influenced by the age of the biofilm as well as the thickness and EPS content in biofilms (Luo et al. 2022). Despite this, several studies have demonstrated the effectiveness of ultraviolet light for inactivating microbial cells in biofilms. Based on the findings of this study, bacteria were able to survive when exposed to UV light for up to 30 min (Table 3), under laboratory conditions. Similarly, Harada and Nascimento (2021) reported the maximum reduction in B. cereus biofilm population on PP occurred after 30 min of UV-C treatment. Gora et al (2019) also reported that UV-C light at 265 nm could inactivate biofilm-bound P. aeruginosa cells; however, biofilm-bound cells proved more resistant to inactivation than planktonic cells, suggesting that biofilms provide some level of protection to the cells in them. The chlorination process is a common treatment used in wastewater treatment plants due to its prolonged germicidal ability and ability to affect biofilm formation at various stages (Ibekwe and Murinda 2019). The inactivation efficiency of chlorine for biofilms depends on its activation strength and diffusibility (Shen et al. 2017). Chlorine penetrates and damages cell membranes, releasing proteins and nucleic acids, and inhibits enzyme activity, thus killing bacteria (Kelkar et al. 2019). It also decreases biofilm hydrophobicity and adhesion by reacting with proteins and polysaccharides (Luo et al. 2022). Buse et al (2019) reported that L. pneumophila biofilm was inactivated on PVC surfaces using free chlorine after 30 min of exposure. In this study, no bacterial growth was observed from biofilms (Table 3) after 10 min of exposure to chlorine. Harada and Nascimento (2021) also reported a reduction in B. cereus biofilm populations on PP after 15 min of treatment with sodium hypochlorite. UV light is a rapid, low maintenance and environmentally safe disinfection method compared to chlorine; however, UV is limited because of photoreactivation and dark repair of bacteria (Wang et al. 2021b). Chlorine is also limited due to its inability to inactivate some chlorine-resistant bacteria, but residual chlorine in water distribution systems after treatment provides residual protection and prevents potential regrowth of bacteria (Wang et al. 2021b; Li et al. 2018).
Conclusion
In conclusion, this study has provided valuable insights into the formation and characteristics of biofilms on microplastic particles (MPs) exposed to wastewater. The results indicate that environmental conditions and nutrient availability play crucial roles in facilitating bacterial attachment and subsequent biofilm formation on MPs. Specifically, the highest biofilm formation occurred at an incubation temperature of 25 °C, under dark and aerobic conditions, suggesting the favorable conditions for biofilm formation by biofilm-forming bacteria. Furthermore, the study revealed that a duration of 3 weeks of MP exposure to wastewater is necessary to achieve maximum bacterial attachment. Moreover, the conversion of ammonia to nitrite and nitrate was observed during different stages of biofilm development under aerobic conditions, indicating the presence of nitrifying bacteria as a dominant microbial community within the biofilm. The physicochemical properties of the MPs were found to influence the concentration of biofilms, with polyethylene (HDPE and LDPE) exhibiting greater biofilm formation compared to polypropylene (PP). Additionally, the surface texture of the MPs played a role, as rough MPs demonstrated a higher concentration of biofilm attachment compared to smooth MPs. The bacterial community attached to the MPs was observed to be more diverse and richer than the bacterial community in the wastewater medium. Proteobacteria was identified as the most abundant phylum throughout the study, followed by Bacteroidetes and Planctomycetes. However, the abundance of Bacteroidetes and Planctomycetes decreased over time. Although the type of plastic influenced the dominant genera attached to each type of MP, Methylotenera, Hydrogenophaga and Rhodanobacter were commonly found on all types of MPs, indicating that the type of MPs does not significantly influence the bacterial community. Furthermore, this study found that both UV and chlorine treatments are effective in inactivating attached biofilms under optimal conditions. Chlorine treatment required a 10-min contact time, while a 30-min exposure to UV light was sufficient for biofilm inactivation. In summary, this study highlights the importance of environmental conditions, nutrient availability and MP properties in facilitating bacterial attachment and biofilm formation. The findings contribute to our understanding of the complex interactions between MPs, bacteria and biofilm formation in wastewater systems, and have implications for the management and treatment of microplastic pollution in aquatic environments.
Recommendations
The current study has made a significant contribution to the field of microbial association with MPs in wastewater; however, some areas could be expanded. A future study could examine how wastewater treatment operational conditions impact biofilm formation in a full-scale environment. In addition to the batch culture conducted in this study, it is recommended that the microbial composition of biofilms be investigated under continuous culture conditions to gain an understanding of biofilm attachment under continuous nutritional supply. Due to the presence of pathogenic species in biofilms, further studies should focus on identifying pathogenic species in biofilms formed on microplastics. Furthermore, it is also necessary to investigate the antibiotic resistance of microorganisms within biofilms as they could be potential carriers of antibiotic-resistant bacteria (ARB) in the environment. Despite the effectiveness of UV and chlorine treatment in deactivating biofilms, it is still possible for biofilms to regrow or reform following tertiary treatment and the use of HPC-based detection alone can be misleading. To gain a deeper understanding of the efficacy of the tertiary methods used in this study, additional research using alternative methods of microbial detection is therefore recommended.
Data and materials availability
All data are presented in the manuscript.
Code availability
Not applicable.
References
Akoğlu A (2020) The effect of some environmental conditions on planktonic growth and biofilm formation by some lactic acid bacteria isolated from a local cheese in Turkey. Biotech Lett 42(3):481–492
Amaral-Zettler LA, Zettler ER, Mincer TJ (2020) Ecology of the plastisphere. Nat Rev Microbiol 18(3):139–151
APHA (2011) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Awolusi OO, Nasr M, Kumari S, Bux F (2018) Principal component analysis for the interaction of nitrifiers and wastewater environments at a full-scale activated sludge plant. Int J Environ Sci Technol 15(7):1477–1490
Bowley J, Baker-Austin C, Porter A, Hartnell R, Lewis C (2021) Oceanic hitchhikers–assessing pathogen risks from marine microplastic. Trends Microbiol 29(2):107–116
Buse HY, Morris BJ, Struewing IT, Szabo JG (2019) Chlorine and monochloramine disinfection of Legionella pneumophila colonizing copper and polyvinyl chloride drinking water biofilms. Appl Environ Microbiol 85(7):e02956-e3018
Chapron L, Peru E, Engler A, Ghiglione JF, Meistertzheim AL, Pruski AM, Purser A, Vétion G, Galand PE, Lartaud F (2018) Macro-and microplastics affect cold-water corals growth, feeding and behaviour. Sci Rep 8(1):1–8
Chen F, Lv M, Ye Y, Miao S, Tang X, Liu Y, Liang B, Qin Z, Chen Y, He Z, Wang Y (2022) Insights on uranium removal by ion exchange columns: the deactivation mechanisms, and an overlooked biological pathway. Chem Eng J 434:134708
de Carvalho CCCR (2017) Biofilms: Microbial strategies for surviving UV exposure. In: Ahmad S (ed) Ultraviolet Light in Human Health, Diseases and Environment, vol 996. Springer, Cham, pp 233–239
De Tender CA, Devriese LI, Haegeman A, Maes S, Ruttink T, Dawyndt P (2015) Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ Sci Technol 49(16):9629–9638
Eckert EM, Di Cesare A, Kettner MT, Arias-Andres M, Fontaneto D, Grossart HP, Corno G (2018) Microplastics increase impact of treated wastewater on freshwater microbial community. Environ Pollut 234:495–502
Feng L, He L, Jiang S, Chen J, Zhou C, Qian ZJ, Hong P, Sun S, Li C (2020) Investigating the composition and distribution of microplastics surface biofilms in coral areas. Chemosphere 252:126565
Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2017) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Kerninon S, Cassone AL, Lambert C, Reveillaud J, Paul-Pont I (2018) Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut 242:614–625
Gora SL, Rauch KD, Ontiveros CC, Stoddart AK, Gagnon GA (2019) Inactivation of biofilm-bound Pseudomonas aeruginosa bacteria using UVC light emitting diodes (UVC LEDs). Water Res 151:193–202
Govaert M, Smet C, Baka M, Ećimović B, Walsh JL, Van Impe J (2018) Resistance of L. monocytogenes and S. Typhimurium towards cold atmospheric plasma as function of biofilm age. Appl Sci 8:2702
Hansen J, Melchiorsen J, Ciacotich N, Gram L, Sonnenschein EC (2021) Effect of polymer type on the colonization of plastic pellets by marine bacteria. FEMS Microbiol Lett 368(5):fnab026
Harada AMM, Nascimento MS (2021) Effect of dry sanitizing methods on Bacillus cereus biofilm. Braz J Microbiol 52(2):919–926
Hoellein TJ, McCormick AR, Hittie J, London MG, Scott JW, Kelly JJ (2017) Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshwater Sci 36(3):491–507
Hossain MR, Jiang M, Wei Q, Leff LG (2019) Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol 59(1):54–61
Huang H, Peng C, Peng P, Lin Y, Zhang X, Ren H (2019) Towards the biofilm characterization and regulation in biological wastewater treatment. Appl Microbiol Biotechnol 103(3):1115–1129
Ibekwe AM, Murinda SE (2019) Linking microbial community composition in treated wastewater with water quality in distribution systems and subsequent health effects. Microorganisms 7(12):660
Karkman A, Do TT, Walsh F, Virta MP (2017) Antibiotic-resistance genes in wastewater. Trends Microbiol 26(3):220–228
Kataky R, Knowles E (2018) Biofilm formation on abiotic surfaces and their redox activity. Curr Opin Electrochem 12:121–128
Kaur S, Roy A (2021) Bioremediation of heavy metals from wastewater using nanomaterials. Environ Dev Sustain 23(7):9617–9640
Kelkar VP, Rolsky C, Pant A, Green MD, Tongay S, Halden RU (2019) Chemical and physical changes of microplastics during sterilization by chlorination. Water Res 163:114871
Kelly JJ, London MG, McCormick AR, Rojas M, Scott JW, Hoellein TJ (2021) Wastewater treatment alters microbial colonization of microplastics. PLoS ONE 16(1):e0244443. https://doi.org/10.1371/journal.pone.0244443
Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI (2018) Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 4(12):e01067
Kirstein IV, Wichels A, Krohne G, Gerdts G (2018) Mature biofilm communities on synthetic polymers in seawater-specific or general? Mar Environ Res 142:147–154
Li W, Zhang J, Wang F, Qian L, Zhou Y, Qi W, Chen J (2018) Effect of disinfectant residual on the interaction between bacterial growth and assimilable organic carbon in a drinking water distribution system. Chemosphere 202:586–597
Li L, He Z, Liang T, Sheng T, Zhang F, Wu D, Ma F (2022) Colonization of biofilm in wastewater treatment: a review. Environ Pollut 293:118514
Lin J, Yan D, Fu J, Chen Y, Ou H (2020) Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res 186:116360
Liu S, Gunawan C, Barraud N, Rice SA, Harry EJ, Amal R (2016) Understanding, monitoring, and controlling biofilm growth in drinking water distribution systems. Environ Sci Technol 50(17):8954–8976
Luo X, Zhang B, Lu Y, Mei Y, Shen L (2022) Advances in application of ultraviolet irradiation for biofilm control in water and wastewater infrastructure. J Hazard Mater 421:126682
Miao L, Wang P, Hou J, Yao Y, Liu Z, Liu S, Li T (2019) Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci Total Environ 650:2395–2402
Miao L, Gao Y, Adyel TM, Huo Z, Liu Z, Wu J, Hou J (2021) Effects of biofilm colonization on the sinking of microplastics in three freshwater environments. J Hazard Mater 413:125370
Nauendorf A, Krause S, Bigalke NK, Gorb EV, Gorb SN, Haeckel M, Wahl M, Treude T (2016) Microbial colonization and degradation of polyethylene and biodegradable plastic bags in temperate fine-grained organic-rich marine sediments. Mar Pollut Bull 103(1–2):168–178
Oberbeckmann S, Osborn AM, Duhaime MB (2017) Microbes on a bottle: substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 11(8):e0159289
Okshevsky M, Gautier E, Farner JM, Schreiber L, Tufenkji N (2020) Biofilm formation by marine bacteria is impacted by concentration and surface functionalization of polystyrene nanoparticles in a species-specific manner. Environ Microbiol Rep 12(2):203–213
Parrish K, Fahrenfeld NL (2019) Microplastic biofilm in fresh-and wastewater as a function of microparticle type and size class. Environ Sci: Water Res Technol 5(3):495–505
Pham DN, Clark L, Li M (2021) Microplastics as hubs enriching antibiotic-resistant bacteria and pathogens in municipal activated sludge. J Hazard Mater Lett 2:100014
Pinto M, Langer TM, Hüffer T, Hofmann T, Herndl GJ (2019) The composition of bacterial communities associated with plastic biofilms differs between different polymers and stages of biofilm succession. PLoS ONE 14(6):e0217165
Rittmann BE (2018) Biofilms, active substrata, and me. Water Res 132:135–145. https://doi.org/10.1016/j.watres.2017.12.043
Rosario DK, Rodrigues BL, Bernardes PC, Conte-Junior CA (2021) Principles and applications of non-thermal technologies and alternative chemical compounds in meat and fish. Crit Rev Food Sci Nutr 61(7):1163–1183
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M (2017) Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett 4(7):258–267
Rummel CD, Lechtenfeld OJ, Kallies R, Benke A, Herzsprung P, Rynek R, Wagner S, Potthoff A, Jahnke A, Schmitt-Jansen M (2021) Conditioning film and early biofilm succession on plastic surfaces. Environ Sci Technol 55(16):11006–11018
Sajjad AA, Mohd YMYB, Vo DN, Tran NH (2020) Biocarriers for biofilm immobilization in wastewater treatments: a review. Environ Chem Lett. https://doi.org/10.1007/s10311-020-01049-y
Saxena P, Joshi Y, Rawat K, Bisht R (2019) Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J Microbiol 59(1):3–12
Schmidt H, Thom M, Wieprecht S, Manz W, Gerbersdorf SU (2018) The effect of light intensity and shear stress on microbial biostabilization and the community composition of natural biofilms. Res Rep Biol 9(9):1–16
Sehar S, Naz I (2016) Role of the biofilms in wastewater treatment. In: Microbial biofilms-importance and applications, p 121–144
Shen Y, Gao J, Li L (2017) Municipal wastewater treatment via co-immobilized microalgal-bacterial symbiosis: Microorganism growth and nutrients removal. Biores Technol 243:905–913
Shen M, Zhu Y, Zhang Y, Zeng G, Wen X, Yi H, Ye S, Ren X, Song B (2019) Micro (nano) plastics: unignorable vectors for organisms. Mar Pollut Bull 139:328–331
Sun J, Dai X, Wang Q, van Loosdrecht MC, Ni BJ (2019) Microplastics in wastewater treatment plants: detection, occurrence and removal. Water Res 152:21–37
Takahashi Y, Fujitani H, Hirono Y, Tago K, Wang Y, Hayatsu M, Tsuneda S (2020) Enrichment of comammox and nitrite-oxidizing Nitrospira from acidic soils. Front Microbiol 11:1737
Tallawi M, Opitz M, Lieleg O (2017) Modulation of the mechanical properties of bacterial biofilms in response to environmental challenges. Biomater Sci 5(5):887–900. https://doi.org/10.1039/c6bm00832a
Totani T, Nishiuchi Y, Tateishi Y, Yoshida Y, Kitanaka H, Niki M, Matsumoto KY, S, (2017) Effects of nutritional and ambient oxygen condition on biofilm formation in Mycobacterium avium subsp. hominissuis via altered glycolipid expression. Sci Rep 7(1):1–12
Townsley L, Yildiz FH (2015) Temperature affects c-di-GMP signalling and biofilm formation in Vibrio cholerae. Environ Microbiol 17:4290–4305
Toyofuku M, Inaba T, Kiyokawa T, Obana N, Yawata Y, Nomura N (2016) Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem 80(1):7–12
Tu C, Zhou Q, Zhang C, Liu Y, Luo, Y (2020) Biofilms of microplastics. In: Microplastics in terrestrial environments, Springer, Cham, 299–317
USEPA (1999) EPA wastewater technology fact sheet: chlorine disinfection. Epa 832- F-99-062, Washington D.C
van Kessel MAHJ, Speth DR, Albertsen M, Nielsen PH, Op Den Camp HJM, Kartal B et al (2015) Complete nitrification by a single microorganism. Nature 528:555–559. https://doi.org/10.1038/nature16459
Wang J, Guo X, Xue J (2021a) Biofilm-developed microplastics as vectors of pollutants in aquatic environments. Environ Sci Technol 55(19):12780–12790
Wang L, Ye C, Guo L, Chen C, Kong X, Chen Y, Shu L, Wang P, Yu X, Fang J (2021b) Assessment of the UV/chlorine process in the disinfection of Pseudomonas aeruginosa: Efficiency and mechanism. Environ Sci Technol 55(13):9221–9230
Wen B, Liu JH, Zhang Y, Zhang HR, Gao JZ, Chen ZZ (2020) Community structure and functional diversity of the plastisphere in aquaculture waters: does plastic color matter? Sci Total Environ 740:140082
Wu X, Pan J, Li M, Li Y, Bartlam M, Wang Y (2019) Selective enrichment of bacterial pathogens by microplastic biofilm. Water Res 165:114979
Yang Y, Liu W, Zhang Z, Grossart HP, Gadd GM (2020) Microplastics provide new microbial niches in aquatic environments. Appl Microbiol Biotechnol 104:6501–6511
Zhang G, Li B, Guo F, Liu J, Luan M, Liu Y, Guan Y (2019) Taxonomic relatedness and environmental pressure synergistically drive the primary succession of biofilm microbial communities in reclaimed wastewater distribution systems. Environ Int 124:25–37
Zhao Y, Gao J, Wang Z, Dai H, Wang Y (2021) Responses of bacterial communities and resistance genes on microplastics to antibiotics and heavy metals in sewage environment. J Hazard Mater 402:123550
Acknowledgements
This study was funded by the Water Research Commission of South Africa (Project No. K5-2952) under the JPIWATER project name "NANOCARRIERS." This study was also partially funded by the South African Research Chair Initiative (SARChI) (UID 84166) and the National Research Foundation of South Africa competitive funding for rated researchers (UID 129358).
Funding
Open access funding provided by Durban University of Technology. This study was funded by the Water Research Commission of South Africa (Project No. K5-2952) as part of the Joint Programme Initiative on Water (JPIWATER) project title “NANOCARRIERS.” The South African Research Chair Initiative (SARChI) (UID 84166) and the National Research Foundation of South Africa (UID 129358) also supported this study financially. We also acknowledge the support from the Durban University of Technology.
Author information
Authors and Affiliations
Contributions
All authors took part in conceptualization, and writing—reviewing and editing; S.R. and I.D.A. were responsible for data collection and writing—original draft preparation; and F.B. and S.K. were involved in supervision.
Corresponding author
Ethics declarations
Conflicts of interest
We have not conflict of interest to declare.
Additional information
Editorial responsibility: Maryam Shabani.
Appendices
Appendix A: Scanning electron microscopy images
Appendix B: Phenol–chloroform DNA extraction protocol
Total DNA was extracted from both wastewater and MPs using the extraction method adapted from Awolusi et al. (2018). Briefly, 500 μL of lysis buffer (1 M Tris–HCl; 0.5 M EDTA; 10% SDS; 5 M) was added to each tube and vortexed thoroughly for 1 min. The tubes were then placed in water bath at 60 °C for 30 min. The temperature was thereafter raised to 65 °C, and the tubes were incubated for a further 2 h. A freeze–thaw step was then carried out, by placing the tubes in ethanol–ice slurry for 3 min and then in a 65 °C water bath for 3 min. This was repeated five times. Thereafter, 500 μL of a freshly made phenol/chloroform/isoamyl alcohol mix (25:24:1) was added to the tubes. These tubes were gently mixed by inversion and centrifuged at 10,000 rpm at for 3 min at 4 °C. The upper aqueous layer was carefully transferred to a fresh set of tubes. Five hundred microliters of chloroform was then added to the tubes. These tubes were gently mixed by inversion and centrifuged at 10,000 rpm at for 3 min at 4 °C. The upper aqueous layer was again carefully transferred to a fresh set of tubes, and the DNA was precipitated by the addition of 600 μL of isopropanol and stored at − 20 °C overnight. The precipitated genomic DNA was pelleted by centrifugation at 12,000 rpm at 4 °C for 20 min, washed with cool 70% ethanol and air-dried for no longer than 15 min. The final DNA pellet was stored in 20 μL 1 × TE buffer (pH 7.5) at − 20 °C. The purity and quantity of the DNA extracts were analyzed by spectrophotometry using the IMPLEN NanoPhotometer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rajcoomar, S., Amoah, I.D., Abunama, T. et al. Biofilm formation on microplastics in wastewater: insights into factors, diversity and inactivation strategies. Int. J. Environ. Sci. Technol. 21, 4429–4444 (2024). https://doi.org/10.1007/s13762-023-05266-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-05266-0