Abstract
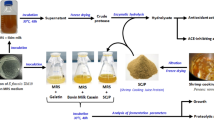
A chitosanase and a protease were purified from the culture supernatant of Serratia sp. TKU016 with shrimp shell as the sole carbon/nitrogen source. The molecular masses of the chitosanase and protease determined by SDS–PAGE were approximately 65 and 53 kDa, respectively. The chitosanase was inhibited completely by Mn2+, but the protease was enhanced by all of tested divalent metals. The optimum pH, optimum temperature, pH stability, and thermal stability of the chitosanase and protease were (pH 7, 50°C, pH 6–7, <50°C) and (pH 8–10, 40°C, pH 5–10, <50°C), respectively. SDS (2 mM) had stimulatory effect on TKU016 protease activity. The result demonstrates that TKU016 protease is SDS-resistant protease and probably has a rigid structure. Besides, TKU016 culture supernatant (2% SPP) incubated for 2 days has the highest antioxidant activity, the DPPH scavenging ability was about 76%. With this method, we have shown that shrimp shell wastes can be utilized and it’s effective in the production of enzymes, antioxidants, peptide and reducing sugar, facilitating its potential use in biological applications and functional foods.






Similar content being viewed by others
References
Banik RM, Prakash M (2004) Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Microbiol Res 159:135–140
Bhaskar N, Sudeepa ES, Rashmi HN, Tamil Selvi A (2007) Partial purification and characterization of protease of Bacillus proteolyticus CFR3001 isolated from fish processing waste and its antibacterial activities. Biores Technol 98:2758–2764
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brurberg MB, Nes IF, Eijsink VGH (1996) Comparative studies of chitinases A and B from Serratia marcescens. Microbiology 142:1581–1589
Frankowski J, Lorito M, Scala F, Schmid R, Berg G, Bahl H (2001) Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol 176:421–426
Green AT, Healy MG, Healy A (2005) Production of chitinolytic enzymes by Serratia marcescens QMB1466 using various chitinous substrates. J Chem Technol Biotechnol 80:28–34
He H, Chen X, Sun C, Zhang Y, Gao P (2006) Preparation and functional evaluation of oligopeptide-enriched hydrolysate from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Biores Technol 97:385–390
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102:196–202
Imoto T, Yagishita K (1971) A simple activity measurement of lysozyme. Agric Biol Chem 35:1154–1156
Kadokura K, Rokutani A, Yamamoto M, Ikegami T, Sugita H, Itoi S, Hakamata W, Oku T, Nishio T (2007) Purification and characterization of Vibrio parahaemolyticus extracellular chitinase and chitin oligosaccharide deacetylase involved in the production of heterodisaccharide from chitin. Appl Microbiol Biotechnol 75:357–365
Kim S, Mendis E (2006) Bioactive compounds from marine processing byproducts-A review. Food Res Intern 39:383–393
Kim HS, Timmis KN, Golyshin PN (2007) Characterization of a chitinolytic enzyme from Serratia sp. KCK isolated from kimchi juice. Appl Microbiol Biotechnol 75:1275–1283
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee KH, Lee PM, Siaw YS, Morihara K (1993) Kinetics of aspartame precursor synthesis catalysed by Pseudomonas aeruginosa elastase. J Chem Technol Biotechnol 56:375–381
Lee YS, Yoo JS, Chung SY, Lee YC, Cho YS, Choi YL (2006) Cloning, purification, and characterization of chitosanase from Bacillus sp. DAU101. Appl Microbiol Biotechnol 73:113–121
Liang TW, Chen YJ, Yen YH, Wang SL (2007) The antitumor activity of the hydrolysates of chitinous materials hydrolyzed by crude enzyme from Bacillus amyloliquefaciens V656. Process Biochem 42:527–534
Lin HY, Chou CC (2004) Antioxidant activities of water-soluble disaccharide chitosan derivatives. Food Res Intern 37:883–889
Nawani NN, Kapadnis BP (2001) One-step purification of chitinase from Serratia marcescens NK1, a soil isolate. J Appl Microbiol 90:803–808
Pinero Estrada JE, Bermejo Bescoś P, Villar del Fresno AM (2001) Antioxidant activity of different fractions of Spriulina platensis protean extract. IL FARMACO 56:497–500
Roberts DP, McKenna LF, Lakshman DK, Meyer SLF, Kong H, Souza JT, Lydon J, Baker CJ, Buyer JS, Chung S (2007) Suppression of dumping-off of cucumber caused by Pythium ultimum with live cells and extracts of Serratia marcescens N4–5. Soil Biol Biochem 39:2275–2288
Romero FJ, Garcia LA, Salas JA, Diaz M, Quiros LM (2001) Production, purification and partial characterization of two extracellular proteases from Serratia marcescens grown in whey. Process Biochem 36:507–515
Sakurai K, Funaguma T, Hara A (1995) Studies on chitinases produced by Serratia sp. isolated from soil. Meijodai Nogakuho 31:21–31
Salamone PR, Wodzinski RJ (1997) Production, purification and characterization of a 50-kDa extracellular metalloprotease from Serratia marcescens. Appl Microbiol Biotechnol 48:317–324
Shikha AS, Darmwal NS (2007) Improve production of alkaline protease from a mutant of alkalophilic Bacillus pantotheneticus using molasses as a substrate. Biores Technol 98:881–885
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem 40:945–948
Su C, Wang D, Yao L, Yu Z (2006) Purification, characterization, and gene cloning of a chitosanase from Bacillus species strain S65. J Agric Food Chem 54:4208–4214
Suzuki K, Sugawara N, Suzuki M, Uchiyama T, Katouno F, Nikaidou N, Watanabe T (2002) Chitinases A, B, and C1 of Serratia marcescens 2170 produced by recombinant Escherichia coli: enzymatic properties and synergism on chitin degradation. Biosci Biotech Biochem 66:1075–1083
Synowiecki J, Al-Khateeb NAAQ (2000) The recovery of protein hydrolysate during enzymatic isolation of chitin from shrimp Crangon crangon processing discards. Food Chem 68:147–152
Tan SC, Khor E, Tan TK, Wong SM (1998) The degree of deacetylation of chitosan: advocating the first derivative UV-spectrophotometry method of determination. Talanta 45:713–719
Tao K, Long Z, Liu K, Tao Y, Liu S (2006) Purification and properties of a novel insecticidal protein from the locust pathogen Serratia marcescens HR-3. Curr Microbiol 52:45–49
Todd EW (1949) Quantitative studies on the total plasmin and trypsin inhibitor of human blood serum. J Exp Med 39:295–308
Ulrich B (1994) Crystal structure of the 50 kDa metallo protease from Serratia marcescens. J Mol Biol 242:244–251
Wang SL, Kao TY, Wang CL, Yen YH, Chern MK, Chen YH (2006) A solvent stable metalloprotease produced by Bacillus sp. TKU004 and its application in the deproteinization of squid pen for β-chitin preparation. Enzyme Microb Technol 39:724–731
Wang SL, Lin HT, Liang TW, Chen YJ, Yen YH, Guo SP (2008a) Reclamation of chitinous materials by bromelain for the preparation of antitumor and antifungal materials. Biores Technol 99:4386–4393
Wang SL, Peng JH, Liang TW, Liu KC (2008b) Purification and characterization of a chitosanase from Serratia marcescens TKU011. Carbohydr Res 343:1316–1323
Wang SL, Lin CL, Liang TW, Liu KC, Kuo YH (2009) Conversion of squid pen by Serratia ureilytica for the production of enzymes and antioxidants. Biores Technol 100:316–323
Xing R, Yu H, Liu S, Zhang W, Zhang Q, Li Z (2005) Antioxidative activity of differently regioselective chitosan sulfates in vitro. Bioorg Med Chem 13:1387–1392
Acknowledgments
This work was supported in part by a Grant from the National Science Council, Taiwan (NSC96-2313-B-032-002-MY3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, SL., Chang, TJ. & Liang, TW. Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials. Biodegradation 21, 321–333 (2010). https://doi.org/10.1007/s10532-009-9303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-009-9303-x