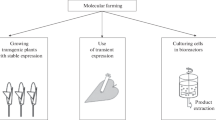
Abstract
Monoclonal antibodies represent the major class of biopharmaceutical products (for therapeutics and diagnostics) with an increasing demand that reaches several tons per year worldwide. Traditional large-scale manufacturing processes are based on stirred tank bioreactors for the growth of Chinese Hamster Ovary cells (CHO) which requires high initial investments and production costs. Therefore, there is an urgent need for alternative production platforms that can at least act as a complement to the over-exploited mammalian fermentation systems. In this perspective, the use of plants for the large-scale production of biopharmaceuticals (‘Molecular farming’) represents an interesting and mature technology that has already proved its benefits in terms of safety, scalability, rapidity and reduced manufacturing costs. Here we discuss the recent advances in the production of monoclonal antibodies (mAbs) in plant-based platforms such as transgenic plants, tissue and cell cultures and transient expression systems.


Similar content being viewed by others
References
Boothe J, Nykiforuk C, Shen Y, Zaplachinski S, Szarka S, Kuhlman P, Murray E, Morck D, Moloney MM (2010) Seed-based expression systems for plant molecular farming. Plant Biotechnol J 8:588–606
Bosch D, Castilho A, Loos A, Schots A, Steinkellner H (2013) N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des 19:5503–5512
Buyel JF, Twyman RM, Fischer R (2017) Very-large-scale production of antibodies in plants: the biologization of manufacturing. Biotechnol Adv 35:458–465
Chen Q, Lai H (2015) Gene delivery into plant cells for recombinant protein production. Biomed Res Int 2015:932161
Chen Q, Lai H, Hurtado J, Stahnke J, Leuzinger K, Dent M (2013) Agroinfiltration as an effective and scalable strategy of gene delivery for production of pharmaceutical proteins. Adv Tech Biol Med 1:103
Circelli P, Donini M, Villani ME, Benvenuto E, Marusic C (2010) Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs 1:221–224
Cox KM, Sterling JD, Regan JT, Gasdaska JR et al (2006) Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 24:1591–1597
De Muynck B, Navarre C, Boutry M (2010) Production of antibodies in plants: status after twenty years. Plant Biotechnol J 8:529–563
Decker EL, Parsons J, Reski R (2014) Glyco-engineering for biopharmaceutical production in moss bioreactors. Front Plant Sci 5:346
Doron L, Segal N, Shapira M (2016) Transgene expression in microalgae-from tools to applications. Front Plant Sci 7:505
Drake PMW, Szeto TH, Paul MJ, Teh AY-H, Ma JK-C (2017) Recombinant biologic products versus nutraceuticals from plants - a regulatory choice? Br J Clin Pharmacol 83:82–87
Ecker DM, Jones SD, Levine HL (2015) The therapeutic monoclonal antibody market. mAbs 7:9–14
Fischer R, Vasilev N, Twyman RM, Schillberg S (2015) High-value products from plants: the challenges of process optimization. Curr Opin Biotechnol 32:156–162
Gavilondo J V, Larrick JW (2000) Antibody engineering at the millennium. BioTechniques 29: 128–32, 134–6, 138 passim
Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y (2006) Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 103:14701–14706
Gleba Y, Klimyuk V, Marillonnet S (2007) Viral vectors for the expression of proteins in plants. Curr Opin Biotechnol 18:134–141
Häkkinen ST, Raven N, Henquet M, Laukkanen M-L et al (2014) Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol Bioeng 111:336–346
Hanania U, Ariel T, Tekoah Y, Fux L et al (2017) Establishment of a tobacco BY2 cell line devoid of plant-specific xylose and fucose as a platform for the production of biotherapeutic proteins. Plant Biotechnol J 15:1120–1129
Hempel F, Maier UG (2012) An engineered diatom acting like a plasma cell secreting human IgG antibodies with high efficiency. Microb Cell Factories 11:126
Hendin HE, Pillet S, Lara AN, Wu C-Y, Charland N, Landry N, Ward BJ (2017) Plant-made virus-like particle vaccines bearing the hemagglutinin of either seasonal (H1) or avian (H5) influenza have distinct patterns of interaction with human immune cells in vitro. Vaccine 35:2592–2599
Hiatt A, Cafferkey R, Bowdish K (1989) Production of antibodies in transgenic plants. Nature 342:76–78
Holtz BR, Berquist BR, Bennett LD, Kommineni VJM, Munigunti RK, White EL, Wilkerson DC, Wong K-YI, Ly LH, Marcel S (2015) Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol J 13:1180–1190
Jansing J, Sack M, Augustine SM, Fischer R, Bortesi L (2018) CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol J. https://doi.org/10.1111/pbi.12981
Jin C, Altmann F, Strasser R, Mach L, Schähs M, Kunert R, Rademacher T, Glössl J, Steinkellner H (2008) A plant-derived human monoclonal antibody induces an anti-carbohydrate immune response in rabbits. Glycobiology 18:235–241
Kelley B (2009) Industrialization of mAb production technology: the bioprocessing industry at a crossroads. mAbs 1:443–452
Kircheis R, Halanek N, Koller I, Jost W, Schuster M, Gorr G, Hajszan K, Nechansky A (2012) Correlation of ADCC activity with cytokine release induced by the stably expressed, glyco-engineered humanized Lewis Y-specific monoclonal antibody MB314. mAbs 4:532–541
Kizhner T, Azulay Y, Hainrichson M, Tekoah Y, Arvatz G, Shulman A, Ruderfer I, Aviezer D, Shaaltiel Y (2015) Characterization of a chemically modified plant cell culture expressed human α-Galactosidase-A enzyme for treatment of Fabry disease. Mol Genet Metabol 114:259–267
Klimyuk V, Pogue G, Herz S, Butler J, Haydon H (2014) Production of recombinant antigens and antibodies in nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr Top Microbiol Immunol 375:127–154
Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL (2010) Transient expression systems for plant-derived biopharmaceuticals. Expert Rev Vaccines 9:859–876
Kopertekh L, Schiemann J (2017) Transient production of recombinant pharmaceutical proteins in plants: evolution and perspectives. Curr Med Chem. https://doi.org/10.2174/0929867324666170718114724
Lico C, Chen Q, Santi L (2008) Viral vectors for production of recombinant proteins in plants. J Cell Physiol 216:366–377
Lim JAC, Patkar A, McDonagh G, Sinclair A, Lucy P (2010) Modeling bioprocess cost: process economic benefits of expression technology based on Pseudomonas fluorescens. BioProcess Int 8:62–70
Lomonossoff GP, D’Aoust M-A (2016) Plant-produced biopharmaceuticals: a case of technical developments driving clinical deployment. Science (New York, N.Y.) 353:1237–1240
Lonoce C, Salem R, Marusic C, Jutras PV, Scaloni A, Salzano AM, Lucretti S, Steinkellner H, Benvenuto E, Donini M (2016) Production of a tumour-targeting antibody with a human-compatible glycosylation profile in N. benthamiana hairy root cultures. Biotechnol J 11:1209–1220
Lonoce C, Marusic C, Morrocchi E, Salzano AM, Scaloni A, Novelli F, Pioli C, Feeney M, Frigerio L, Donini M (2018) Enhancing the secretion of a glyco-engineered anti-CD20 scFv-Fc antibody in hairy root cultures. Biotechnol J. https://doi.org/10.1002/biot.201800081
Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, van Dolleweerd C, Mostov K, Lehner T (1995) Generation and assembly of secretory antibodies in plants. Science (New York, N.Y.) 268:716–719
Ma JK-C, Drossard J, Lewis D, Altmann F et al (2015) Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J 13:1106–1120
Magy B, Tollet J, Laterre R, Boutry M, Navarre C (2014) Accumulation of secreted antibodies in plant cell cultures varies according to the isotype, host species and culture conditions. Plant Biotechnol J 12:457–467
Marusic C, Pioli C, Stelter S, Novelli F, Lonoce C, Morrocchi E, Benvenuto E, Salzano AM, Scaloni A, Donini M (2018) N-glycan engineering of a plant-produced anti-CD20-hIL-2 immunocytokine significantly enhances its effector functions. Biotechnol Bioeng 115:565–576
Mathieu-Rivet E, Kiefer-Meyer M-C, Vanier G, Ovide C, Burel C, Lerouge P, Bardor M (2014) Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front Plant Sci 5:359
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci USA 100:438–442
Mehrotra S, Srivastava V, Rahman LU, Kukreja AK (2015) Hairy root biotechnology—indicative timeline to understand missing links and future outlook. Protoplasma 252:1189–1201
Mercx S, Smargiasso N, Chaumont F, De Pauw E, Boutry M, Navarre C (2017) Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 Cells by a Multiplex CRISPR/cas9 strategy results in glycoproteins without plant-specific glycans. Front Plant Sci 8:403
Montero-Morales L, Steinkellner H (2018) Advanced plant-based glycan engineering. Front Bioeng Biotechnol 6:81
Mor TS (2015) Molecular pharming’s foot in the FDA’s door: protalix’s trailblazing story. Biotechnol Lett 37:2147–2150
Navarre C, Smargiasso N, Duvivier L, Nader J, Far J, De Pauw E, Boutry M (2017) N-Glycosylation of an IgG antibody secreted by Nicotiana tabacum BY-2 cells can be modulated through co-expression of human β-1,4-galactosyltransferase. Transgenic Res 26:375–384
Pillet S, Aubin É, Trépanier S, Bussière D, Dargis M, Poulin J-F, Yassine-Diab B, Ward BJ, Landry N (2016) A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol (Orlando, Fla.) 168:72–87
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Biotechnol Adv 28:910–918
PREVAIL II Writing Group, Multi-National PREVAIL II Study Team, Davey RT, Dodd L, Proschan MA, Neaton J et al (2016) A randomized, controlled trial of ZMapp for ebola virus infection. NE ngl J Med 375:1448–1456
Qiu X, Wong G, Audet J, Bello A et al (2014) Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature 514:47–53
Rademacher T (2013) Method for the generation and cultivation of a plant cell pack. Patent WO2013/113504
Rademacher T, Sack M, Arcalis E, Stadlmann J et al (2008) Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J 6:189–201
Raven N, Rasche S, Kuehn C, Anderlei T et al (2015) Scaled-up manufacturing of recombinant antibodies produced by plant cells in a 200-L orbitally-shaken disposable bioreactor. Biotechnol Bioeng 112:308–321
Reski R, Parsons J, Decker EL (2015) Moss-made pharmaceuticals: from bench to bedside. Plant Biotechnol J 13:1191–1198
Sainsbury F, Sack M, Stadlmann J, Quendler H, Fischer R, Lomonossoff GP (2010) Rapid transient production in plants by replicating and non-replicating vectors yields high quality functional anti-HIV antibody. PLoS ONE 5:e13976
Santos RB, Abranches R, Fischer R, Sack M, Holland T (2016) Putting the spotlight back on plant suspension cultures. Front Plant Sci 7:297
Saxena P, Thuenemann EC, Sainsbury F, Lomonossoff GP (2016) Virus-derived vectors for the expression of multiple proteins in plants. Methods Mol Biol (Clifton, N.J.) 1385:39–54
Schillberg S, Raven N, Fischer R, Twyman RM, Schiermeyer A (2013) Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr Pharm Des 19:5531–5542
Shamriz S, Ofoghi H (2016) Outlook in the application of Chlamydomonas reinhardtii chloroplast as a platform for recombinant protein production. Biotechnol Genet Eng Rev 32:92–106
Shen J-S, Busch A, Day TS, Meng X-L et al (2016) Mannose receptor-mediated delivery of moss-made α-galactosidase A efficiently corrects enzyme deficiency in Fabry mice. J Inherit Metabol Dis 39:293–303
Sheshukova EV, Komarova TV, Dorokhov YL (2016) Plant factories for the production of monoclonal antibodies. Biochemistry 81:1118–1135
Shukla AA, Wolfe LS, Mostafa SS, Norman C (2017) Evolving trends in mAb production processes. Bioeng Trans Med 2:58–69
Sijmons PC, Dekker BM, Schrammeijer B, Verwoerd TC, van den Elzen PJ, Hoekema A (1990) Production of correctly processed human serum albumin in transgenic plants. Bio/technology (Nature Publishing Company) 8:217–221
Steinkellner H, Castilho A (2015) N-Glyco-engineering in plants: update on strategies and major achievements. Methods Mol Biol (Clifton, N.J.) 1321:195–212
Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H (2008) Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 6:392–402
Tekoah Y, Shulman A, Kizhner T, Ruderfer I et al (2015) Large-scale production of pharmaceutical proteins in plant cell culture-the Protalix experience. Plant Biotechnol J 13:1199–1208
Tusé D, Ku N, Bendandi M, Becerra C et al (2015) Clinical safety and immunogenicity of tumor-targeted, plant-made Id-KLH conjugate vaccines for follicular lymphoma. Biomed Res Int 2015:648143
Vanier G, Lucas P-L, Loutelier-Bourhis C, Vanier J, Plasson C, Walet-Balieu M-L et al (2017) Heterologous expression of the N-acetylglucosaminyltransferase I dictates a reinvestigation of the N-glycosylation pathway in Chlamydomonas reinhardtii. Sci Rep 7:10156
Vanier G, Stelter S, Vanier J, Hempel F, Maier UG, Lerouge P, Ma J, Bardor M (2018) Alga-made anti-Hepatitis B antibody binds to human Fcγ receptors. Biotechnol J 13:e1700496
Vasilev N, Grömping U, Lipperts A, Raven N, Fischer R, Schillberg S (2013) Optimization of BY-2 cell suspension culture medium for the production of a human antibody using a combination of fractional factorial designs and the response surface method. Plant Biotechnol J 11:867–874
Weintraub JA, Hilton JF, White JM, Hoover CI, Wycoff KL, Yu L, Larrick JW, Featherstone JDB (2005) Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res 39:241–250
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root-inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:33–44
Wongsamuth R, Doran PM (1997) Production of monoclonal antibodies by tobacco hairy roots. Biotechnol Bioeng 54:401–415
Xu J, Zhang N (2014) On the way to commercializing plant cell culture platform for biopharmaceuticals: present status and prospect. Pharm Bioprocess 2:499–518
Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ (2012) Green factory: plants as bioproduction platforms for recombinant proteins. Biotechnol Adv 30:1171–1184
Yao J, Weng Y, Dickey A, Wang KY (2015) Plants as Factories for human pharmaceuticals: applications and challenges. Int J Mol Sci 16:28549–28565
Yusibov V, Kushnir N, Streatfield SJ (2016) Antibody production in plants and green algae. Annu Rev Plant Biol 67:669–701
Zischewski J, Sack M, Fischer R (2016) Overcoming low yields of plant-made antibodies by a protein engineering approach. Biotechnol J 11:107–116
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donini, M., Marusic, C. Current state-of-the-art in plant-based antibody production systems. Biotechnol Lett 41, 335–346 (2019). https://doi.org/10.1007/s10529-019-02651-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-019-02651-z