Abstract
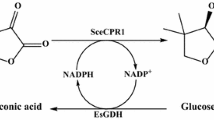
A substrate-coupled biocatalytic process was developed based on the reactions catalyzed by an NADPH-dependent sorbose reductase (SOU1) from Candida albicans in which ethyl 4-chloro-3-oxobutanoate (COBE) was reduced to (S)-4-chloro-3-hydroxybutanoate [(S)-CHBE], while NADPH was regenerated by the same enzyme via oxidation of sugar alcohols. (S)-CHBE yields of 1,140, 1,150, and 780 mM were obtained from 1,220 mM COBE when sorbitol, mannitol, and xylitol were used as co-substrates, respectively. Optimization of COBE and sorbitol proportions resulted in a maximum yield of (S)-CHBE (2,340 mM) from 2,500 mM COBE, and the enantiomeric excess was 99.6 %. The substrate-coupled system driven by SOU1 maintained a stable pH and a robust intracellular NADPH circulation; thus, pH adjustment and addition of extra coenzymes were unnecessary.



Similar content being viewed by others
References
An MD, Cai P, Yan M, Hao N, Wang SS, Liu H, Li Y, Xu L (2012) A novel reductase from Candida albicans for the production of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 76(6):1210–1212. doi:10.1271/bbb.120048
Greenberg JR, Price NP, Oliver RP, Sherman F, Rustchenko E (2005) Candida albicans SOU1 encodes a sorbose reductase required for l-sorbose utilization. Yeast 22(12):957–969. doi:10.1002/yea.1282
Karanewsky DS, Badia MC, Ciosek CP Jr, Robl JA, Sofia MJ, Simpkins LM, DeLange B, Harrity TW, Biller SA, Gordon EM (1990) Phosphorus-containing inhibitors of HMG-CoA reductase. 1,4-[(2-arylethyl)hydroxyphosphinyl]-3-hydroxy-butanoic acids: a new class of cell-selective inhibitors of cholesterol biosynthesis. J Med Chem 33(11):2952–2956
Kizaki N, Yasohara Y, Hasegawa J, Wada M, Kataoka M, Shimizu S (2001) Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Appl Microbiol Biotechnol 55(5):590–595
Wang LJ, Li CX, Ni Y, Zhang J, Liu X, Xu JH (2011) Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor. Bioresour Technol 102(14):7023–7028. doi:S0960-8524(11)00560-8
Yamamoto H, Matsuyama A, Kobayashi Y (2002) Synthesis of ethyl (R)-4-chloro-3-hydroxybutanoate with recombinant Escherichia coli cells expressing (S)-specific secondary alcohol dehydrogenase. Biosci Biotechnol Biochem 66(2):481–483
Yamamoto H, Mitsuhashi K, Kimoto N, Matsuyama A, Esaki N, Kobayashi Y (2004) A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Biosci Biotechnol Biochem 68(3):638–649
Yamamoto H, Mitsuhashi K, Kimoto N, Kobayashi Y, Esaki N (2005) Robust NADH-regenerator: improved alpha-haloketone-resistant formate dehydrogenase. Appl Microbiol Biotechnol 67(1):33–39. doi:10.1007/s00253-004-1728-x
Ye Q, Cao H, Yan M, Cao F, Zhang Y, Li X, Xu L, Chen Y, Xiong J, Ouyang P, Ying H (2010a) Construction and co-expression of a polycistronic plasmid encoding carbonyl reductase and glucose dehydrogenase for production of ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresour Technol 101(17):6761–6767. doi:S0960-8524(10)00576-6
Ye Q, Li X, Yan M, Cao H, Xu L, Zhang Y, Chen Y, Xiong J, Ouyang P, Ying H (2010b) High-level production of heterologous proteins using untreated cane molasses and corn steep liquor in Escherichia coli medium. Appl Microbiol Biotechnol 87(2):517–525. doi:10.1007/s00253-010-2536-0
Ye Q, Ouyang P, Ying H (2011) A review-biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate ester: recent advances and future perspectives. Appl Microbiol Biotechnol 89(3):513–522. doi:10.1007/s00253-010-2942-3
Yu MA, Wei YM, Zhao L, Jiang L, Zhu XB, Qi W (2007) Bioconversion of ethyl 4-chloro-3-oxobutanoate by permeabilized fresh brewer’s yeast cells in the presence of allyl bromide. J Ind Microbiol Biotechnol 34(2):151–156. doi:10.1007/s10295-006-0179-z
Acknowledgments
This work was supported by the National Basic Research Program of China (No. 2011CBA00804) and the Innovation Fund for Doctor Degree Dissertation in Nanjing University of Technology (No. BSCX200809).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, P., An, M., Xu, L. et al. Development of a substrate-coupled biocatalytic process driven by an NADPH-dependent sorbose reductase from Candida albicans for the asymmetric reduction of ethyl 4-chloro-3-oxobutanoate. Biotechnol Lett 34, 2223–2227 (2012). https://doi.org/10.1007/s10529-012-1029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1029-x