Abstract
Motile aeromonads are an opportunistic and heterogenic bacteria that cause motile Aeromonas septicemia (MAS) which is accompanied with variety of clinical signs and lesions in fish. It has been found that their outer membrane proteins (OMPs) are highly immunogenic and may be used as a vaccine candidate. The present study was undertaken to evaluate OMP protection against Aeromonas hydrophila (A. hydrophila) strain ATCC 7966 (A5 and A8) and A. hydrophila strain DSM 30,187 (A6). Pathogenicity tests revealed that out of 12 isolated strains, seven were highly pathogenic, three were moderate, and two were avirulent. SDS-PAGE analysis of OMPs showed several protein bands (7–22) of different molecular weights ranging from 14 to 346 kDa. The common protein band was 39 kDa. It seems that pathogenicity and the number of protein bands are related in the highly pathogenic strains. Challenged A. hydrophila for Nile tilapia (O. niloticus) previously actively immunized with OMPs (A4, A5, A6, A8, and A11) showed a significant increase in relative percent survival (RPS) ranging from 50 to 100%. Immunological parameters such as immunoglobulin M (IgM), antioxidants (SOD, GSH, and CAT), and nitric oxide (NO) and expression of pro-inflammatory cytokines, interleukin 1β (IL-1β), and tumor necrosis factor-α (TNFα) showed a significant increase in OMP-immunized fish. Challenged A. hydrophila for O. niloticus previously passively immunized with OMPs (A4, A5, A6, A8, and A11) in fish-raised sera showed a significant increase in RPS (20 to 100%) in the vaccinated groups. It is concluded that OMPs from Aeromonas spp. have been identified as suitable target for protection development, especially in the case of heterogenic bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Egypt is first in Africa and sixth globally in aquaculture production, specifically in tilapia production, which is the main freshwater cultured fish (USDA 2022). The aquaculture industry has been subjected to some emerging and re-emerging outbreaks, coupled with insufficient diagnostic labs and diagnostic tools may be the main cause of high losses (Dadar et al. 2017; Faisal et al. 1989). Motile Aeromonas septicemia (MAS) is a common serious disease caused byAeromonas spp. These are the most important bacteria for the aquaculture industry, mainly Aeromonas hydrophila (A. hydrophila) (Faisal et al. 1989). An emerging pathotype of Aeromonads have been responsible for widespread severe economic losses in the aquaculture industry (Faisal et al. 1989). Type III secretion system (T3SS), lipopolysaccharide (LPS), outer membrane proteins (OMPs), pili and flagella, and extracellular factors like exo-toxin, aerolysins, hemolysins, enterotoxin, and siderophore are some of the virulence factors that contribute to the complex and multifaceted pathogenicity of Aeromonads infection (Chopra et al. 2000; Peatman et al. 2018; Pablos et al. 2009). Fish manifested signs of MAS such as severe hemorrhagic dermatitis, orbital cellulitis, and ocular rupture (Janda and Abbott 2010). BecauseA. hydrophilaisolates are heterogenic and the bacteria are becoming more resistant to a variety of antibiotics, it is challenging to stop disease outbreaks caused by this bacterium (Baumgartner et al. 2017). Thus, immunoprophylaxis is a risk-free way to stop outbreaks in aquaculture. But there is no commercial vaccination forA. hydrophilaat this time (Daskalov 2006). This may be because of the insufficient capacity to meet the efficacy, safety, or cost-effectiveness necessary for the vaccine formulations that have been tried to date (Fang et al. 2004). Bacterial OMPs have exposed epitopes on their cell surface that enable them to readily engage with the host immune system, making them highly immunogenic and promising candidates for vaccine development (Clark and Cassidy-Hanley 2005; Lin et al. 2002). Fang et al. (2004) showed significant protection against two isolates ofA. hydrophila in blue gourami, Trichogaster richopterus, immunized with a recombinant 43 kDa OMP. More recently, a recombinant OMP (37 kDa) of A. hydrophilawas produced and proved to be immunogenic in rohu carp (Thirumalaikumar et al. 2022).
Understanding the fish immune system is crucial for the creation of complementary therapies and preventative measures against disease outbreaks (Khushiramani et al. 2007). Immunoglobulin M and cytokine genes are crucial for controlling fish immunological function (Aoki and Ikuo 2006). After other antioxidant molecules run out, antioxidant enzymes become essential in the fight against oxidative stress brought on by toxicants (Lindenstrom et al. 2004). These enzymes are essential in oxidative stress to deal with free radicals, causing multiple disruptions. They include superoxide dismutase (SOD), which converts superoxide anion radicals to hydrogen peroxide, and catalase (CAT), which reduces hydrogen peroxide to water (Martinez-Alvares et al. 2005; Pinto et al. 2003; Tripathi et al. 2006). To test the immunoprotective effectiveness of Aeromonad isolates of outer membrane proteins (OMPs) onOreochromis niloticus, a potential vaccine was being developed. It is concluded that OMPs from Aeromonas spp. have been identified as suitable targets for protection development, especially in the case of heterogenic bacteria.
Materials and methods
Fish and experimental conditions
Batch of apparently healthy O. niloticus(30 ± 5 g body weight) used in this study were obtained from certified private fish farm in Kafr El Sheikh and transferred to the laboratory of Aquatic Animal Medicine Department, Faculty of Veterinary Medicine, Benha University, Moshtohor, Egypt, in large tanks supplied with oxygen with no previous history of disease underwent external inspection before being assigned to experimental groups then also examined for health status immediately upon arrival, including abnormal movement, faded or darkened pigmentation, skin lesions, fin and tail erosion, and external fungi or parasites (Amlacher 1970).
For 2 weeks, fish were housed in 750-L fiber glass tanks for acclimation to the lab conditions. Dechlorinated tap water and an air pump were provided for every tank. A static system was used where the water was renewed at a rate of 20% every 24 h and uneaten foods were removed every day.
The water temperature was adjusted to 26 ± 2 °C, dissolved oxygen content to 6 ± 0.5 mg/L, ammonia concentration 0.53 ± 0.07 mg/L, and pH to 7 ± 0.2. Fish were fed a basal diet at 4% of their body weight twice a day. Fish samples were sacrificed at random, and their organs were tested for parasites, mycoses, and bacteria to ensure they were free from any pathogens as described by Austin and Austin (2007).
Aeromonad strains
Twelve well-identified aeromonads bacterial spp. named (A1, A2, A3, A4, A5, A6, A7, A8, A9, A10, A11, and A12) isolated from clinically diseased freshwater fishes (Oreochromis niloticus, Mugil cephalus, and Clarias gariepinus) (Matter et al. 2018) were used in this study. The preserved bacterial isolates at −80 °C (Hollander and Nell 1954) were also identified again using API 20E and molecular identification to ensure that the preserve culture was pure and were not contaminated then were inoculated into 20 ml Tryptic Soya Broth (TSB) media and grown at 28 °C for 24-h incubation and then different doses were adjusted for challenging fish. The bacterial growth was harvested in sterile physiological saline NaCl (0.9%) and bacterial cell concentrations (1.5, 3, 6, 9 × 108cells/ml) was adjusted using spectrophotometer with absorbance 0.1, 0.257, 0.451, and 0.582, respectively, at wavelength 620 nm (El- Asely et al. 2014).
Phylogenetic analyses for identification of challenged A. hydrophila strains
Seven selected PCR products as representative strains were subjected to sequencing to identify A. hydrophila strains. Using the QIAquick PCR Purification Kit (QIAGEN, USA), the PCR products were purified. Purified PCR products were sequenced on an AB 3500 Genetic Analyzer using a Big Dye Terminator Kit, in accordance with the manufacturer’s instructions. The sequences that were produced were examined using Applied Biosystem software (version 6). The resulting sequence was examined against the database of strain types and published, accepted prokaryotic nomenclature using the neighbor joining blast tree and the Basic Local Alignment Search Tool (BLAST) tool from the National Center for Biotechnology Information (NCBI).
Assessment of Aeromonad isolate virulence
The intensity of Aeromonad isolate virulence was evaluated as follows; a total of 500 fish were divided into 13 groups. Each group from the first 12 groups was divided into 4 subgroups. Ten fish in each subgroup were injected i.p. (0.2 mL) with different bacterial cell concentration (0.3, 0.6, 1.2, 1.8 × 108 cells/fish) for the 12 Aeromonad isolates. Group No. 13 was kept as control (n= 20 fish) which was inoculated with sterile phosphate buffer saline (PBS, pH 7.5). On the day of the challenge, the experimental fish received no feed. After the challenge the following day, food was provided twice a day for the duration of the trial at a rate of 4% of body weight. For 7 days following the injection, the clinical signs and mortality rates in both the infected and control groups were documented every day. After being re-isolated from several organs (liver, kidneys, and spleen), the bacterial strains were fully identified as previously described (Matter et al. 2018). The restored bacteria from -80°c freezing should be passage several times into fish (Nile tilapia) to restore their virulance due to long freezing led to loss the virulance of previosly isolated bacteria.
Extraction of OMPs
The bacterial isolates were cultured on brain-heart infusion broth at 28 °C for 18 h in order to extract outer membrane proteins. The cells were then recovered by centrifugation at 2550 × g for 20 min at 4 °C. Once the cells reached an OD620 (1.5), they were again suspended in 5 mL of 0.5 TRIS HCl (pH 7.4) and sonicated five times for a duration of 1 min on ice. Centrifugation was used to remove cell debris for 10 min at 4 °C at 2550 × g. A 20% N-lauroyl sarcosine sodium salt (Sigma, USA) was then added in 0.5 mL and allowed to sit at room temperature for 30 min. After being centrifuged at 17,650 × g for an hour at 4 °C, the OMPs were twice cleaned with PBS, suspended in fresh PBS and frozen at −20 °C (Abbass et al. 2010).
SDS-PAGE
Electrophoresis for 12 Aeromonad OMPs was performed using pre-stained high molecular weight standard markers (Sigma, USA). OMPs were run on a 12% (w/v) acrylamide separating gel after being placed onto a 4% stacking gel. Gels were de-stained with 10% (v/v) acetic acid and 40% (v/v) methanol after being stained with Coomassie brilliant blue (R-250), Sigma, USA. Dice similarity index was calculated for every isolate as described by Laemmli (1970) and dendrogram was then constructed using (Advanced American biotechnology, UPG MA, USA).
Immunoprotective efficiency of Aeromonad OMPs antigen in O. niloticus
Safety test
Safety of crude OMPs antigen on O. niloticus(30 ± 5 g body weight) was performed according to Midtlyng (2016) with minor modification briefly; three fish were IP inoculated with 0.1 ml of OMPs antigen suspension (20 µg protein) of the highly virulentA. hydrophila (A6) in triplicate, keeping three fish in separate aquarium as control which were injected IP with sterile saline. All fish were placed under investigation for 7 days, with recording of clinical signs, developed lesions, and mortality rates.
Active immunization of O. niloticus with OMPs antigens
Preparation of the OMPs
Adequate amount of separated crude OMPs from the five representative strains of Aeromonads (four were separated from O. niloticus isolates: A4, A5, A6, and A11). While the fifth was separated from C. gariepinus, (A8) was used. Protein concentration in OMPs suspension was carried out by the method of Lowry et al. (1951), and preserved at −20 till use.
Preparation of challenge bacteria
The bacteria virulent strains (A5, A6, and A8) were grown on brain-heart infusion broth at 28 °C for 18 h. The bacterial cells were collected by centrifugation at 2550 × g for 20 min at 4 °C and adjusted for 9 × 108cells/mL (El- Asely et al. 2014).
Experimental design
A total of 288 O. niloticus (30 ± 5 g body weight) from the acclimated stock were divided into six groups (16 fish each in triplicate) and placed in well-prepared aquaria, One group was kept as control (control positive) and the remaining five groups (A, B, C, D, and E) were injected 0.1 mL of emulsified OMPs of A4, A5, A6, A8, and A11, respectively, with Freund’s complete adjuvant (FCA) (first dose). Thirty-four days post injection (Poobalane 2007), fish received booster dose at the same dose (0.1 mL) of emulsified OMPs with Freund’s incomplete adjuvant (FIA) for 17 days (Poobalane 2007). The control groups (negative and positive) were injected with PBS. Fifty-one days post injection (at the end of the experiment), serum and tissue samples (liver and spleen) were collected from 18 fish in each group that were properly preserved till use. For evaluation of OMPs protection, the remaining 30 fish from each group were challenged IP with 0.2 mL of 1.8 × 108 cells/fish for the three A. hydrophila isolates (A5, A6, and A8) at the opposite side where they had been injected with the booster injection. Briefly, the challenged bacteria were injected as the following: group (B) was challenged with its corresponding A. hydrophila (A5), group (C) was challenged with its corresponding A. hydrophila (A6), and group (D) was challenged with its corresponding A. hydrophila (A8). Groups (A), (E), and (cont. positive) were challenged with A6.
Calculation of relative percentage survival (RPS)
After 28 days post challenge, RPS was calculated using the formula of Amend (1981).
Effect of active immunization on immune parameters
Evaluation of serum immunoglobulin M (IgM)
Serum IgM levels in immunized fish was measured spectrophotometrically following the protocol of ELISA kits (Cusabio Biotech Co. Ldt, USA).
Determination of antioxidant enzymes activities and nitric oxide
Superoxide dismutase enzyme activity (SOD) and catalase activity (CAT) were measured spectrophotometrically at 510 nm and calculated according to Fossati et al. (1980). Glutathione reductase enzyme activity (GSH) was determined colorimetrically at 405 nm and calculated according to Satoh (1978). Nitric oxide enzyme activity (NO) was estimated according to Rajaraman et al. (1998). All enzyme activities were measured by using Bio-diagnostic kits (Egypt).
Gene expression estimation
After being immunized with Aeromonad OMPs, O. niloticus immune response was evaluated by assessing the expression of genes that encode the pro-inflammatory cytokines TNF-α and IL-1β. To investigate variations in IL1-β and TNF-α expression, quantitative real-time PCR was performed on specific genes extracted from the spleens of all treated groups as well as the control group. Using the RNeasy Mini Kit (Qiagen, USA) and the manufacturer’s instructions, total RNA was extracted. Gene-specific primers were employed in qPCR to measure variations in the expression levels of the genes and listed in Table 1. The cycling conditions of SYBR green real time PCR were performed according to Quantitect SYBR green PCR kit.
The amplification profile was carried out as follows: 40 cycles of primary denaturation at 94°C for 15 s, optimal primer annealing temperature at 62°C for 30 s, and extension at 72°C for 30 s were performed after the initial denaturation at 94°C for 5 min. One cycle of the dissociation curve consists of 1 min of secondary denaturation at 94°C, 1 min of primer annealing at 62°C, and 1 min of final denaturation at 94°C. The software strata gene MX3005P was used to determine CT values and amplification curves. The CT of each sample was compared with that of the control group in order to quantify the variance of gene expression on the RNA of the various samples according to the “ΔΔCt” method stated by Yuan et al. (2006), using the following ratio: (2−DDct), whereas ΔΔCt = ΔCt reference – ΔCt target.
Passive immunization of O. niloticus with anti-OMPs serum
Experimental design
A total of 90 O. niloticus (30 ± 5 g body weight) from the acclimated stock were divided into six groups: five fish each in triplicate and placed in well-prepared aquaria. One group is the control (cont.) and the remaining 5 groups (1, 2, 3, 4, and 5) were injected 0.1 mL of O. niloticus sera raised against the OMPs (A4, A5, A6, A8, and A11, respectively). Cont. group was injected with serum collected from non-vaccinated O. niloticus. For the evaluation of OMPs antisera protection, after 24 h, all fish were challenged with i.p. injection of 0.2 mL of 1.8 × 108 cells/fish for the three A. hydrophila isolates (A5, A6, and A8) at the opposite side where they had been injected with the antiserum (Lafrentz et al. 2003). Briefly, the challenged bacteria were injected as the following: group (2) was challenged with its correspondingA. hydrophila (A5) and group (4) was challenged with A. hydrophila (A8). Group (3) was challenged with its corresponding A. hydrophila (A6), while groups (1), (5), and (cont.) were challenged with (A6).
Calculation of relative percentage survival (RPS)
After 28 days, the relative percentage survival (RPS) was calculated as in active immunization.
Statistical analysis
Statistical significance across groups was analyzed using one-way analysis of variance with post hoc Duncan tests, using SPSS 16.0 software to determine the significant changes in different parameters. A p-value ˂ 0.05 was the threshold for significance (Yuan et al. 2006).
Results
Virulence of Aeromonad isolates
Starting from the 1st day until the 3rd day, the mortality rates in infected fish showed a high rate, and then decreased gradually or stopped till the 7th day post-injection. Out of the 12 tested Aeromonad isolates, 7 isolates (A2, A3, A4, A5, A6, A11, and A12) were found to be virulent strains, resulting in 80–100% mortality when injected with a dose 1.8 × 108 cells/fish. The remainder of the three strains (A7, A8, and A9) showed moderate virulence, reaching 40, 60, and 60%, respectively, while A1 and A10 recorded no mortalities (avirulent strains) (Table 2).
SDS-PAGE analysis of OMPs
Considerable differences were found in the number of OMP bands from the tested Aeromonads examined by SDS-PAGE, which ranged from 7 to 22 with molecular weights ranging from 14 to 346 kDa (Fig. 1). The most common bands were 22, 37, and 39 kDa in most of the strains. The dominant band (39 kDa) was seen in 8 out of the 12 isolates. Dendrogram analysis revealed that 12 aeromonad isolates were divided into 2 clusters. The first cluster includes A1, A8, A9, A10, and A12, which are then divided into two sub-clusters. The first sub-cluster includes A8, A9, A10, and A12, and the second sub-cluster includes only A1. The first sub-cluster was divided into two groups. The first group includes A8, A9, and A10, with no difference between A8 and A10, while the second group includes only A12 (Fig. 2). The second cluster includes A2, A3, A4, A5, A6, A7, and A11, and then is divided into two sub-clusters. The first sub-cluster includes A2, A3, and A11, with no difference between A2 and A3, and the second sub-cluster includes A4, A5, A6, A7, then divided into two groups. The first group includes A5, A6, and A7, with no difference between A5 and A6, while the second group includes only A4 (Fig. 2).
Coomassie blue stained SDS-PAGE analysis of OMPs extracted from Aeromonas spp. Lanes (1–12); M, marker; lane (1, 2): Aeromonas sp. OMPs protein bands isolated from Mugil cephalus ; lane (3, 4, 5, 6, 7, 11, and 12): Aeromonas sp. OMPs protein bands isolated from Oreochromis niloticus; lane ( 8, 9, and 10): Aeromonas spp. OMPs protein bands isolated from Clarias gariepinus. M indicates the migration of the protein molecular weight (KDa) marker
Relationship between pathogenicity and OMP profile
Highly pathogenic strains (A2, A3, A4, A5, A6, A11, and A12) contained OMP bands (18, 17, 15, 17, 22, 14, and 13), respectively, in corresponding strains.
Phylogenetic analyses for identification of challenged A. hydrophila strains
Based upon the sequence analysis of the 7 Aeromonad isolates using housekeeping genes gyrB, A5, A6, and A8 were identified as A. hydrophila, showing high phylogenetic relatedness (Fig. 3), and A3 and A4 are A. finlandiensis with 100% similarity, while A7 was identified as A. bestiarum (Fig. 3) and was assembled in a separate tree, with only 50% similarity with other isolates, and A1 was recognized as A. rivipollensis, which showed 50% similarity with A3 and A4 (Fig. 3).
Active immunization of O. niloticus with OMP antigens
Safety test
The safety test revealed that the injected crude OMPs were safe; no clinical signs or lesions were reported.
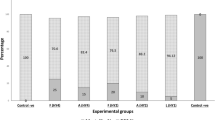
Evaluation of the protective effect of OMP antigen on O. niloticus (RPS)
The relative percentage survival (RPS) 4 weeks post-challenge with pathogenic A. hydrophila isolates showed that group (B) was 60% when challenged with A. hydrophila (A5) while group (C) was 90% RPS when challenged with A. hydrophila (A6). Group (D) was 50% RPS when challenged with A. hydrophila (A8). In addition, groups (A), (E), and (cont.) had 100, 100, and 0% RPS, respectively, when challenged with the highly virulent strain A. hydrophila (A6) (Fig. 4).
Effect of active immunization on immune parameters
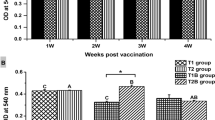
Effect on IgM levels
O. niloticus-immunized groups (A, B, C, D, and E) with crude OMPs showed a significant increase (p < 0.05) in serum IgM levels compared to the control group, with the highest levels recorded in groups (A), (C), and (E) and the lowest detected in group (D) (Fig. 5).
Effect of active immunization on antioxidant enzymes and nitric oxide (NO)
It was recorded that the SOD activity of all OMP-immunized groups (A, B, C, D, and E) was significantly increased (p < 0.05) in comparison with the control group (Table 3).The catalase activity of O. niloticus-immunized groups (A, C, and E) showed a significant (p < 0.05) increase compared to the control group, but no significant changes were recorded in the catalase activity in groups (B) and (D) (Table 3). Glutathione reductase enzyme activity (GSH) did not show significant changes in all immunized groups compared to control (Table 3). NO levels showed a significant increase (p < 0.05) in immunized groups (A, C, and E), while no significant changes were observed in the values of NO in groups (B) and (D) (Table 3).
Effect of active immunization on expression of IL-1β and TNF-α in the spleen of O. niloticus
IL-1β expression was significantly upregulated in treated groups (A), (C), and (E) than in the control group. But groups (B) and (D) exhibited a slight upregulation of IL-1β (Fig. 6). The same results were observed in TNFα expression, which showed significant upregulation in groups immunized (A), (C), and (E) compared to the control group. No significant changes were recorded in the expression level of TNFα in the spleen tissue of the O. niloticus immunized group (B) (Fig. 6).
Passive immunization
Sera rose against crude OMPs used to passively immunize fish. Groups that received sera against A4, A6, and A11 and were challenged with A. hydrophila (A6) showed the highest degree of protection with RPS 100, 80, and 80%, respectively, while fish immunized with sera against A5 and A8 showed the same lowest protection with RPS 20% when challenged with each corresponding strain (Fig. 7).
Discussion
Aeromonads are thought to be a significant bacterial issue for the advancement of aquaculture. According to certain theories, aquatic environments are significant aeromonad reservoirs, and these bacteria can proliferate and flourish in a range of limnetic conditions (Lafrentz et al. 2003).
Mortality in the current investigation started on the first day following the challenge. After injecting each of the 12 tested isolates with 1.8 × 108 cells/mL, seven of them proved to be virulent, and A. hydrophila(A6) was the most virulent strain with a 100% mortality rate. In the same respect, Rasmia Hanafy (2005), El Deen et al. (2014), and Basma et al. (2016) observed that mortality rates among the experimentally infectedO. niloticus varied according to the strains of A. hydrophila, with mortalities ranging from 50 to 100% by i.p. injection of each with 0.2 mL of 9 × 108 cells/mL suspension. A variety of virulence factors are produced by virulent strains and Aeromonasspecies that have varying mortalities. These virulence factors can be found in two forms: extracellular products such as cytotoxic, hemolytic, and enterotoxic proteins, and cell-associated structures such as pili, flagella, outer membrane proteins, lipopolysaccharide, and capsules (Handfield et al. 1996). Because of their virulence, the bacteria are able to infiltrate, enter, multiply, and harm the tissues of their hosts. They can also evade the host’s defensive mechanism and spread, ultimately leading to the host’s death (Abdullah et al. 2003; Pang et al. 2015).
The OMP profiles of the Aeromonads isolated from three freshwater fishes revealed three bands in several isolates and weighed around 22, 37, and 39 kDa, with the major band being 39 kDa. Thangaviji et al. (2012) found a band around 37 kDa in OMP profiles in threeAeromonassp. strains isolated from carp and goldfish. Meanwhile, Khushiramani et al. (2008) isolated 3–4 high-intensity OMP bands from 40 strains ofAeromonas spp. with a range of 25–45 KDa.
The relatedness between pathogenicity and the number of protein bands showed that highly pathogenic strains (A2, A3, A4, A5, A6, A11, and A12) contained several OMP bands (18, 17, 15, 17, 22, 14, and 13 bands, respectively) corresponding to strains. Using SDS gel electrophoresis, Peng et al. (2014) evaluated isolates ofA. hydrophila against eight antimicrobial drugs and categorized the samples according to the outer membrane protein (OMP) pattern. The discovery by the scientists that the strains exhibiting comparable OMP profiles also exhibited comparable resistance suggests the possibility of other variables contributing to A. hydrophilaantimicrobial resistance. The gram-negative bacteria’s outer membrane serves as a molecular filter for substances that are hydrophilic (Hancock 1997). Permeability changes in the outer membrane are one of the reasons bacteria have resistance to antibiotics (Chevalier et al. 2000). The development of pores by OMPs is one of their most crucial roles in maintaining the permeability of the outer membrane. This membrane’s permeability is a major factor in how susceptible it is to antibiotics. Antibiotic resistance may be influenced by modifications to OMP profiles (Delcour 2009).
Seven strains of Aeromonads that were isolated from different fish species and collected from different locations were evaluated for genetic variability and relatedness using UPGMA analysis, which revealed heterogeneity and distance within the phylogenic tree. Similarly, six isolates of Aeromonads were identified by Sarkar et al. (2012) as beingA. hydrophila; these isolates came from various sources where genetic variability and distance within the phylogenic tree were seen.
It has been demonstrated that fish that have been actively vaccinated with different Aeromonad isolate OMPs exhibit varying degrees of protection when challenged with the relevant pathogens, with RPS reaching 87.5–100%. Even though only a small number of fish were used in the research, OMPs could provide a good vaccine candidate to combat this pathogen. Nearly similar results were obtained by Poobalane (2007), who found that 50 kDa protein induced protection in goldfish againstA. hydrophilain the active immunization trial. Additionally, Thangaviji et al. (2012) discovered thatC. auratus vaccinated with recombinant OMP demonstrated a higher percentage of survival (more than 70%) following the virulent A. hydrophila challenge. Additionally, they discovered that C. auratus vaccinated with A. hydrophila OMP and the OMP gene demonstrated protection and that the OMP vaccine was particularly influential in improving the survival rate (80%) following the virulent A. hydrophila challenge.
Estimation of immunoglobulin M (IgM) revealed that groups were immunized with A4, A6, and A11OMPs antigens, which have immunogenic effects in O. niloticus.These findings are supported by the results of Thangaviji et al. (2011), who foundC. auratus vaccinated with A. hydrophila OMP and the OMP gene showed protection, and the OMP vaccine was highly influenced to improve immunological parameters. The authors added that bacterial OMP reduced the specific bacteria after the A. hydrophila challenge by increasing the production of a higher antibody, and the higher antibody production level may suppress the growth of the A. hydrophilaload. Similarly, Yin et al. (2008) recorded that protection ofA. hydrophila with OMP + herbal (Lonicera japonica and Ganoderma lucidum) immunoadjuvant helped to increase the immunoglobulin M level in O. niloticus against the A. hydrophila challenge.
In OMPs inoculated with O. niloticus, there was a considerable increase in SOD, CAT activity, and NO levels. Halyna et al. (2014) reported nearly similar findings, demonstrating that the OMPs vaccine against furunculosis exposed trout to increased levels of oxidative stress and antioxidant enzyme responses (catalase, glutathione reductase, glutathione peroxidase, and nitric oxide) when compared to the control group. These responses were more severe in the liver than in other tissues. Similar findings of elevated SOD activity in carp tissues after exposure to the OMP vaccination have been reported (Ural 2013; Oruc 2010).
The immune response of O. niloticus after immunization with Aeromonads OMPs was assessed by measuring the expression of genes encoding IL-1β and TNFα. IL-1β and TNFα expression were significantly upregulated in all immunized groups compared with control. The higher upregulation is found in the group immunized with A4, A6, and A11, with the maximum level in the group receiving A11. Zhang et al. (2018) reported nearly identical results: the group immunized with recombinantLactobacillus casei expressing OmpAI had significantly (p< 0.05) upregulated expression of IL-10, IL-1β, IFN-γ, and TNFα genes as compared to control groups, suggesting that inflammatory response and cell immune response were triggered. These cytokines are observable upon stimulation with OMPs, LPs, or microorganisms. TNF-α and IL-8 are two of the inflammatory cytokines that are induced when IL1-β is expressed. Furthermore, Jiang et al. (2016) reported that the expression profiles of several immune-related genes at various points after immunization demonstrated variable upregulations of immune-related genes, such as the pro-inflammatory cytokine IL-1β, the chemokine IL-10, and the liver and spleen of common carp injected in the vaccines with the live attenuatedA. hydrophila strain XX1LA and the formalin-killed A. hydrophila (FKA). The protection seen in passively immunized O. niloticus raised sera against A. hydrophilawith RPS reaches 80–100%. These findings were supported by the results of Poobalane (Poobalane 2007), who found that sera raised against OMP protein bands of 50 kDa induce protection in goldfish againstA. hydrophila. Also, Zhang et al. (2016) found that the anti-OMP serum could provide the fish with immediate protection againstA. hydrophila with RPS 100%, as evidenced by passive immunization.
Conclusion
Aeromonas hydrophila OMPs are promising candidates for vaccine development because they are highly immunogenic, protect against motile Aeromonas septicemia (MAS) in Oreochromis niloticus, and promote protection against the disease.
Data availability
No datasets were generated or analysed during the current study.
References
Abbass A, Sharifuzzaman SM, Austin B (2010) Cellular components of probiotics control yersinia ruckeri infection in rainbow trout, oncorhynnchus mykiss (Walbaum). J Fish Dis 33(1):31–37
Abdullah A, Hart C, Winstanley C (2003) Molecular characterization and distribution of Virulence Associated genes amongst Aeromonas isolates from Libya. J Appl Microbiol 95:1001–1007
Amend DF (1981) Potency testing of fish vaccines. In: Anderson DP, Hennessen H (eds) Fish biologics: sero diagnostics and vaccines. Developments in biological standardisation. Karger, Basel, pp 447–454
Amlacher E (1970) Text book of fish diseases. Gustav Fisher, Tena DDR
Aoki T, Ikuo H (2006) Immune relevant genes of Japanese lounder, Paralichthys olivaceus. Comp Biochem Physiol Part D Genomics Proteom 1:115–121
Austin B, Austin DA (2007) Bacterial fish pathogens, Disease of farmed and wild fish, 4th edn. Springer Praxis, Godalming
Basma D, Ola B, Samy K, Mahmoud T (2016) Molecular characterization, Antimicrobial susceptibility and salt tolerance of Aeromonas hydrophila from Fresh, Brackish and Marine fishes. Alexandria J Vet Sci 48(2):46–53
Baumgartner WA, Ford L, Hanson L (2017) Lesions caused by virulent Aeromonas hydrophila in farmed catfish (Ictalurus punctatus and I. punctatus×I. Furcatus) in Mississippi. J Vet Diagn Investig 29:747–751
Chevalier J, Pages JM, Eyraud A, Mallea M (2000) Membrane permeability modifications are involved in antibiotic resistance in Klebsiella pneumoniae. Biochem Biophys Res Commun 274:496–499
Chopra AK, Xu XJ, Ribardo D, Gonzalez M, Kuhl K, Peter-son JW, Houston CW (2000) The cytotoxic enterotoxin of Aeromonas hydrophila induces pro-inflammatory cytokine production and activates arachidonic acid metabolism in macrophages. Infect Immun 68:2808–2818
Clark TG, Cassidy-Hanley D (2005) Recombinant subunit vaccines: potential and constraints. In: Midtlyng PJ (ed) Progress in fish vaccinology. Karger, Basel, pp 153–164
Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthik K, Tiwari R, Joshi SK (2017) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev Fish Sci Aquac 25(3):184–217
Daskalov H (2006) The importance of Aeromonas hydrophilain food safety. Food Control 17:474–483
Delcour AH (2009) Outer membrane permeability and antibiotic resistance. Biochim et Biophys Acta (BBA)-Proteins Proteom 1794:808–816
El- Asely AM, Abbass AA, Austin B (2014) Honey bee pollen improves growth, immunity and protection of Nile tilapia (Oreochromis niloticus) against infection with Aeromonas hydrophila. Fish Shellfish Immunol 40(2):500–506
El Deen N, Deen AE, Sohad MD, Azza HM, Hakim A (2014) S. studies on Aeromonas hydrophila in cultured Oreochromis niloticus at Kafr El-Sheikh Governorate, Egypt with reference to histopathological alterations in some vital organs. World J Fish Mar Sci 6(3):233–240
Faisal M, Popp W, Refai M (1989) Aeromonas hydrophila-related septicemia in the Nile tilapia (Oreochromis niloticus). Berl Munch Tierarztl Wochenschr 102:87–93
Fang HM, Ge R, Sin YM (2004) Cloning, characterization and expression of Aeromonas hydrophila major adhesion. Fish Shellfish Immunol 16:645–658
Fossati P, Prencipe L, Berti G (1980) Use of 3, 5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymatic assay of uric acid in serum and urine. Clin Chem 26:227–223
Janda JM, Abbott SL (2010) The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73
Halyna T, Natalia K, Joanna G, Anastasiia A (2014) Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol Biochem 40:1289–1300
Hancock R (1997) The bacterial outer membrane as a drug barrier. Trends Microbiol 5:37–42
Handfield M, Simard P, Couillard M, Letarte R (1996) Aeromonas hydrophila isolated from food and drinking water: Hemagglutination, Hemolysis, and cytotoxicity for a human intestinal cell line (HT-29). Appl Environ Microbiol 62:3459–3461
Hollander DH, Nell EE (1954) Improved preservation of Treponema pallidum and other bacteria by freezing with glycerol. Appl Microbiol 2(3):164–170
Jiang X, Zhang C, Zhao Y, Kong X, Pei C, Li L, Nie G, Li X (2016) Immune effects of the vaccine of live attenuated Aeromonas hydrophila screened by rifampicin on common carp (Cyprinus carpio L). Vaccine 1–6
Khushiramani R, Girisha SK, Bhowmick PP, Karunasagar I (2007) Cloning and expression of an outer membrane protein omp TS of Aeromonas hydrophila and study of immunogenicity in fish. Protein Expr Purif 51:303–307
Khushiramani R, Girisha SK, Bhowmick PP, Karunasagar I (2008) Prevalence of different outer membrane proteins in isolates of Aeromonas species. World J Microbiol Biotechnol 24:2263–2268
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophages T4. Nature 227:680–685
Lafrentz BR, LaPatra SE, Jones GR, Cain KD (2003) Passive immunization of rainbow trout, Oncorhynchus mykiss (Walbaum), aganist Flavobacterium psychrophilum, the causative agent of bacterial coldwater disease and rainbow trout fry syndrome. J Fish Dis 26:377–384
Lin J, Huang S, Zhang Q (2002) Outer membrane proteins: key players for bacterial adaptation in host niches. Microbes Infect 4:325–331
Lindenstrom T, Secombes CJ, Buchmann K (2004) Expression of immune response genes in rainbow trout skin induced by Gyrodactylus derjavini infections. Vet Immunol Immunopathol 97:137–148
Lowry OH, Rosenberough NJ, Farr AL, Randal RJ (1951) Protein measurement with folinphenol reagent. J BioChem 193:265–275
Martinez-Alvares RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15:75–88
Matter AF, El Asely AM, Shaheen AA, El-Gawad A, El-Abd EA, Abbass AA (2018) Phenotypic and molecular characterization of bacterial pathogens isolated from diseased freshwater fishes. Int J Fisheries Aquat Stud 6(2):34–41
Midtlyng PJ (2016) Methods for Measuring Efficacy, Safety and Potency of Fish vaccines. In: Adams A (ed) Fish vaccines. Birkhäuser advances in Infectious diseases. Springer, Basel. https://doi.org/10.1007/978-3-0348-0980-1_6
Oruc EO (2010) Oxidative stress, steroid hormone concentrations and acetylcholinesterase activity in Oreochromis niloticus exposed to Aeromonads OMPs. Pestic Biochem Physiol 96:160–166
Pablos M, Rodríguez-Calleja JM, Santos JA, Otero A, García-López ML (2009) Occurrence of motile Aeromonas in municipal drinking water and distribution of genes encoding virulence factors. Int J Food Microbiol 135:158–164
Pang M, Jiang J, Xie X, Wu Y, Dong Y, Kwok AHY, Zhang W, Yao, Lu C, Leung FC, Liu (2015) Y novel insights into the pathogenicity of epidemic Aeromonas hydrophila ST251 clones from comparative genomics. Sci Rep 5:09833. https://doi.org/10.1038/srep09833
Peatman E, Mohammed H, Kirby A, Shoemaker CA, Yildirim-Aksoy M, Beck BH (2018) Mechanisms of pathogen virulence and host susceptibility in virulent Aeromonas hydrophila infections of channel catfish (Ictalurus punctatus). Aquaculture 482:1–8
Peng G, Wang N, Cheng-ping L (2014) Antimicrobial susceptibility and characterization of Outer Membrane Proteins of Aeromonas hydrophila isolated in China. J Integr Agric 13(4):911–917
Pinto E, Sigaud-Kutner TCS, Leitao MAS, Okamoto OK, Morse D, Colepicolo P (2003) Heavy metal-induced oxidative stress in algae. J Phycol 39:1008–1018
Poobalane S (2007) Aeromonas hydrophila Vaccine Development Using Immunoproteomics. A thesis submitted to the University of Stirling for the degree of Doctor of Philosophy; 1- 217
Rajaraman V, Nonnecke BJ, Franklin St, Hamell DC, Horst RL (1998) Effect of vitamin A and E on nitric oxide production by mononuclear leukocytes from neonatal calves fed milk replacer. J Dairy Sci 81:3278–3285
Rasmia Hanafy MAL (2005) Some studies on septicemic diseases of cultured freshwater fishes. M. V. Sc. Thesis, Benha Univ., Egypt
Sarkar A, Saha M, Roy P (2012) Identification and typing of Aeromonas hydrophila through 16S rDNA-PCR fingerprinting. J Aquac Res Dev 3:6
Satoh K (1978) Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. J Clin Chem Acta 90:37–43
Thangaviji V, Michael Babu M, Velmurugan S, Kumanan T, Anand SB, Gunasekaran P, Citarasu T (2011) Virulence factors and molecular cloning of outer membrane protein (OMP) gene from virulent Aeromonas hyrophila isolated from infected gold fish Carassius auratus. Bangladesh J Microbiol 28:70–75
Thangaviji V, Michael Babu M, Velmurugan S, Kumanan T, Anand SB, Gunasekaran P, Citarasu T (2012) Immunization with the Aeromonas OMP provides Protection against Aeromonas hydrophila in Goldfish (Carassius auratus). Microb Biochem Technol 4(2):045–049
Thirumalaikumar E, Sathishkumar R, Vimal S, Babu MM, Uma G, Lusiastuti AM, Citarasu T (2022) Efficacy of recombinant subunit OMP and hly vaccines against Aeromonas hydrophila in Rohu (Labeo rohita). J Fish Dis 45(10):1581–1592
Tripathi BN, Mehta SK, Amar A, Gaur JP (2006) Oxidative stress in Scenedesmus sp. during short-and long-term exposure to Cu2 + and Zn2+. Chemosphere 62:538–544
Ural MS (2013) Aeromonads OMPs -induced changes in oxidant/antioxidant status and haematological parameters of Cyprinus carpio: ameliorative effect of lycopene. Chemosphere 90(7):2059–2064
USDA (United States Department of Agriculture) (2022) An overview of the aquaculture industry in Egypt. Report Number: EG2022-0003
Yin G, Ardo L, Jeney Z, Xu P, Jeney G (2008) Chinese herbs (Lonicera japonica and Ganoderma lucidum) enhance non-specificimmune response of tilapia, Oreochromis niloticus, and protection against Aeromonas hydrophila Diseases in Asian Aquaculture VI, Fish Health section, vol 500. Asian Fisheries Society, Manila, pp 269–282
Yuan GC, Ping M, Zhong W, Liu J (2006) s. statistical assessment of the global regulatory role of histone acetylation in Saccharomyces cerevisiae. Genome Biol 7:R70–R72
Zhang DX, Kang YH, Chen L, Siddiqui SA, Wang CF, Qian AD, Shan (2018) X. F. oral immunization with recombinant Lactobacillus casei expressing OmpAI confers protection against Aeromonas veronii challenge in common carp, Cyprinus carpio. Fish Shellfish Immunol 72:552–563
Zhang D, Xu ZD, Shoemaker D (2016) Experimental induction of motile Aeromonas septicemia in channel catfish (Ictalurus punctatus) by waterborne challenge with virulent Aeromonas hydrophila. Aquac Rep 3:18–23
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AM, AE, AS and AA designed the study, AM performed the experimental part, and AM, AE, AS and AA drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical acceptance
The Committee of Animals Welfare and Research Ethics of Benha University’s Faculty of Veterinary Medicine, Egypt, provided recommendations for doing this research.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matter, A.F., El Asley, A.M., Abbass, A.A. et al. Utilizing outer membrane proteins (OMPs) as a vaccine to manage motile Aeromonas septicemia (MAS) in Nile tilapia (Oreochromis niloticus) with evaluating the vaccine immunoprotective effectiveness. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01511-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01511-6