Abstract
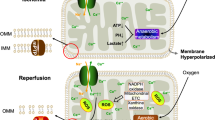
Stroke is the leading cause of adult disability and death worldwide. Mitochondrial dysfunction has been recognized as a marker of neuronal death during ischemic stroke. Maintaining the function of mitochondria is important for improving the survival of neurons and maintaining neuronal function. Damaged mitochondria induce neuronal cell apoptosis by releasing reactive oxygen species (ROS) and pro-apoptotic factors. Mitochondrial fission and fusion processes and mitophagy are of great importance to mitochondrial quality control. This paper reviews the dynamic changes in mitochondria, the roles of mitochondria in different cell types, and related signaling pathways in ischemic stroke. This review describes in detail the role of mitochondria in the process of neuronal injury and protection in cerebral ischemia, and integrates neuroprotective drugs targeting mitochondria in recent years, which may provide a theoretical basis for the progress of treatment of ischemic stroke. The potential of mitochondrial-targeted therapy is also emphasized, which provides valuable insights for clinical research.



Similar content being viewed by others
References
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7(3):243–253. https://doi.org/10.2174/187152708784936608
Henninger N, Fisher M (2016) Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl Stroke Res 7(4):284–293. https://doi.org/10.1007/s12975-015-0444-4
Wang X, Asahi M, Lo EH (1999) Tissue type plasminogen activator amplifies hemoglobin-induced neurotoxicity in rat neuronal cultures. Neurosci Lett 274(2):79–82. https://doi.org/10.1016/s0304-3940(99)00682-5
Segura T, Calleja S, Jordan J (2008) Recommendations and treatment strategies for the management of acute ischemic stroke. Expert Opin Pharmacother 9(7):1071–1085. https://doi.org/10.1517/14656566.9.7.1071
Andrabi SS, Parvez S, Tabassum H (2020) Ischemic stroke and mitochondria: mechanisms and targets. Protoplasma 257(2):335–343. https://doi.org/10.1007/s00709-019-01439-2
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen X, Cai Y, Li L, Wang WE, Zeng C (2015) Mitochondrial DNA oxidative damage contributes to cardiomyocyte ischemia/reperfusion-injury in rats: cardioprotective role of lycopene. J Cell Physiol 230(9):2128–2141. https://doi.org/10.1002/jcp.24941
Crack PJ, Taylor JM (2005) Reactive oxygen species and the modulation of stroke. Free Radic Biol Med 38(11):1433–1444. https://doi.org/10.1016/j.freeradbiomed.2005.01.019
Muller FL, Liu Y, Van Remmen H (2004) Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem 279(47):49064–49073. https://doi.org/10.1074/jbc.M407715200
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13. https://doi.org/10.1042/bj20081386
Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL (2009) Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787(11):1395–1401. https://doi.org/10.1016/j.bbabio.2009.06.009
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35(9):505–513. https://doi.org/10.1016/j.tibs.2010.04.002
Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N (2008) Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14(6):458–470. https://doi.org/10.1016/j.ccr.2008.11.003
Yang Y, Song Y, Loscalzo J (2007) Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A 104(26):10813–10817. https://doi.org/10.1073/pnas.0702027104
Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A 101(47):16419–16424. https://doi.org/10.1073/pnas.0407396101
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M (2005) Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120(5):649–661. https://doi.org/10.1016/j.cell.2004.12.041
Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 95(1):316–321. https://doi.org/10.1073/pnas.95.1.316
Waagepetersen HS, Sonnewald U, Gegelashvili G, Larsson OM, Schousboe A (2001) Metabolic distinction between vesicular and cytosolic GABA in cultured GABAergic neurons using 13C magnetic resonance spectroscopy. J Neurosci Res 63(4):347–355. https://doi.org/10.1002/1097-4547(20010215)63:4%3c347::Aid-jnr1029%3e3.0.Co;2-g
Cheng A, Hou Y, Mattson MP (2010) Mitochondria and neuroplasticity. ASN Neuro 2(5):e00045. https://doi.org/10.1042/an20100019
Bakthavachalam P, Shanmugam PST (2017) Mitochondrial dysfunction - Silent killer in cerebral ischemia. J Neurol Sci 375:417–423. https://doi.org/10.1016/j.jns.2017.02.043
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Twig G, Shirihai OS (2011) The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14(10):1939–1951. https://doi.org/10.1089/ars.2010.3779
Westermann B (2008) Molecular machinery of mitochondrial fusion and fission. J Biol Chem 283(20):13501–13505. https://doi.org/10.1074/jbc.R800011200
Hoppins S, Lackner L, Nunnari J (2007) The machines that divide and fuse mitochondria. Annu Rev Biochem 76:751–780. https://doi.org/10.1146/annurev.biochem.76.071905.090048
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta 1777(9):1092–1097. https://doi.org/10.1016/j.bbabio.2008.05.001
Ono T, Isobe K, Nakada K, Hayashi JI (2001) Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28(3):272–275. https://doi.org/10.1038/90116
Anzell AR, Maizy R, Przyklenk K, Sanderson TH (2018) Mitochondrial Quality Control and Disease: Insights into Ischemia-Reperfusion Injury. Mol Neurobiol 55(3):2547–2564. https://doi.org/10.1007/s12035-017-0503-9
Song BD, Schmid SL (2003) A molecular motor or a regulator? Dynamin’s in a class of its own. Biochemistry 42(6):1369–1376. https://doi.org/10.1021/bi027062h
Boldogh IR, Pon LA (2006) Interactions of mitochondria with the actin cytoskeleton. Biochim Biophys Acta 1763(5–6):450–462. https://doi.org/10.1016/j.bbamcr.2006.02.014
Hollenbeck PJ, Saxton WM (2005) The axonal transport of mitochondria. J Cell Sci 118(Pt 23):5411–5419. https://doi.org/10.1242/jcs.02745
Amiri M, Hollenbeck PJ (2008) Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol 68(11):1348–1361. https://doi.org/10.1002/dneu.20668
Zhao YX, Cui M, Chen SF, Dong Q, Liu XY (2014) Amelioration of ischemic mitochondrial injury and Bax-dependent outer membrane permeabilization by Mdivi-1. CNS Neurosci Ther 20(6):528–538. https://doi.org/10.1111/cns.12266
Suen DF, Norris KL, Youle RJ (2008) Mitochondrial dynamics and apoptosis. Genes Dev 22(12):1577–1590. https://doi.org/10.1101/gad.1658508
Wu S, Zhou F, Zhang Z, Xing D (2011) Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. Febs j 278(6):941–954. https://doi.org/10.1111/j.1742-4658.2011.08010.x
de Arriba G, Calvino M, Benito S, Parra T (2013) Cyclosporine A-induced apoptosis in renal tubular cells is related to oxidative damage and mitochondrial fission. Toxicol Lett 218(1):30–38. https://doi.org/10.1016/j.toxlet.2013.01.007
Ferrari LF, Chum A, Bogen O, Reichling DB, Levine JD (2011) Role of Drp1, a key mitochondrial fission protein, in neuropathic pain. J Neurosci 31(31):11404–11410. https://doi.org/10.1523/jneurosci.2223-11.2011
Kobashigawa S, Suzuki K, Yamashita S (2011) Ionizing radiation accelerates Drp1-dependent mitochondrial fission, which involves delayed mitochondrial reactive oxygen species production in normal human fibroblast-like cells. Biochem Biophys Res Commun 414(4):795–800. https://doi.org/10.1016/j.bbrc.2011.10.006
Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Gräber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E (2006) Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. Embo j 25(16):3900–3911. https://doi.org/10.1038/sj.emboj.7601253
Grohm J, Kim SW, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19(9):1446–1458. https://doi.org/10.1038/cdd.2012.18
Lee H, Yoon Y (2014) Mitochondrial fission: regulation and ER connection. Mol Cells 37(2):89–94. https://doi.org/10.14348/molcells.2014.2329
Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282(15):11521–11529. https://doi.org/10.1074/jbc.M607279200
Qi X, Disatnik MH, Shen N, Sobel RA, Mochly-Rosen D (2011) Aberrant mitochondrial fission in neurons induced by protein kinase C{delta} under oxidative stress conditions in vivo. Mol Biol Cell 22(2):256–265. https://doi.org/10.1091/mbc.E10-06-0551
Horbay R, Bilyy R (2016) Mitochondrial dynamics during cell cycling. Apoptosis 21(12):1327–1335. https://doi.org/10.1007/s10495-016-1295-5
Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR (2012) Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15(2):186–200. https://doi.org/10.1016/j.cmet.2012.01.009
Han XJ, Lu YF, Li SA, Kaitsuka T, Sato Y, Tomizawa K, Nairn AC, Takei K, Matsui H, Matsushita M (2008) CaM kinase I alpha-induced phosphorylation of Drp1 regulates mitochondrial morphology. J Cell Biol 182(3):573–585. https://doi.org/10.1083/jcb.200802164
Chang CR, Blackstone C (2007) Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282(30):21583–21587. https://doi.org/10.1074/jbc.C700083200
Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E (2008) Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9(7):505–518. https://doi.org/10.1038/nrn2417
Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A 105(41):15803–15808. https://doi.org/10.1073/pnas.0808249105
Xu S, Wang P, Zhang H, Gong G, Gutierrez Cortes N, Zhu W, Yoon Y, Tian R, Wang W (2016) CaMKII induces permeability transition through Drp1 phosphorylation during chronic β-AR stimulation. Nat Commun 7:13189. https://doi.org/10.1038/ncomms13189
Dimmer KS, Scorrano L (2006) (De)constructing mitochondria: what for? Physiology (Bethesda) 21:233–241. https://doi.org/10.1152/physiol.00010.2006
Escobar-Henriques M, Anton F (2013) Mechanistic perspective of mitochondrial fusion: tubulation vs. fragmentation. Biochim Biophys Acta 1833 (1):162-175. doi:https://doi.org/10.1016/j.bbamcr.2012.07.016
Benard G, Karbowski M (2009) Mitochondrial fusion and division: Regulation and role in cell viability. Semin Cell Dev Biol 20(3):365–374. https://doi.org/10.1016/j.semcdb.2008.12.012
Rojo M, Legros F, Chateau D, Lombès A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115(Pt 8):1663–1674
Liesa M, Palacín M, Zorzano A (2009) Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89(3):799–845. https://doi.org/10.1152/physrev.00030.2008
Chernomordik LV, Kozlov MM (2005) Membrane hemifusion: crossing a chasm in two leaps. Cell 123(3):375–382. https://doi.org/10.1016/j.cell.2005.10.015
Meglei G, McQuibban GA (2009) The dynamin-related protein Mgm1p assembles into oligomers and hydrolyzes GTP to function in mitochondrial membrane fusion. Biochemistry 48(8):1774–1784. https://doi.org/10.1021/bi801723d
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456(7222):605–610. https://doi.org/10.1038/nature07534
Züchner S, De Jonghe P, Jordanova A, Claeys KG, Guergueltcheva V, Cherninkova S, Hamilton SR, Van Stavern G, Krajewski KM, Stajich J, Tournev I, Verhoeven K, Langerhorst CT, de Visser M, Baas F, Bird T, Timmerman V, Shy M, Vance JM (2006) Axonal neuropathy with optic atrophy is caused by mutations in mitofusin 2. Ann Neurol 59(2):276–281. https://doi.org/10.1002/ana.20797
Peng C, Rao W, Zhang L, Wang K, Hui H, Wang L, Su N, Luo P, Hao YL, Tu Y, Zhang S, Fei Z (2015) Mitofusin 2 ameliorates hypoxia-induced apoptosis via mitochondrial function and signaling pathways. Int J Biochem Cell Biol 69:29–40. https://doi.org/10.1016/j.biocel.2015.09.011
Zhang L, He Z, Zhang Q, Wu Y, Yang X, Niu W, Hu Y, Jia J (2014) Exercise pretreatment promotes mitochondrial dynamic protein OPA1 expression after cerebral ischemia in rats. Int J Mol Sci 15(3):4453–4463. https://doi.org/10.3390/ijms15034453
Klionsky DJ (2008) Autophagy revisited: a conversation with Christian de Duve. Autophagy 4(6):740–743. https://doi.org/10.4161/auto.6398
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462(2):245–253. https://doi.org/10.1016/j.abb.2007.03.034
Zhang X, Yan H, Yuan Y, Gao J, Shen Z, Cheng Y, Shen Y, Wang RR, Wang X, Hu WW, Wang G, Chen Z (2013) Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9(9):1321–1333. https://doi.org/10.4161/auto.25132
Luo T, Park Y, Sun X, Liu C, Hu B (2013) Protein misfolding, aggregation, and autophagy after brain ischemia. Transl Stroke Res 4(6):581–588. https://doi.org/10.1007/s12975-013-0299-5
Wang Y, Han R, Liang ZQ, Wu JC, Zhang XD, Gu ZL, Qin ZH (2008) An autophagic mechanism is involved in apoptotic death of rat striatal neurons induced by the non-N-methyl-D-aspartate receptor agonist kainic acid. Autophagy 4(2):214–226. https://doi.org/10.4161/auto.5369
Narendra D, Tanaka A, Suen DF, Youle RJ (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183(5):795–803. https://doi.org/10.1083/jcb.200809125
Chen Y, Dorn GW 2nd (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340(6131):471–475. https://doi.org/10.1126/science.1231031
Wauer T, Simicek M, Schubert A, Komander D (2015) Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature 524(7565):370–374. https://doi.org/10.1038/nature14879
Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13(5):589–598. https://doi.org/10.1038/ncb2220
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524(7565):309–314. https://doi.org/10.1038/nature14893
Wu W, Lin C, Wu K, Jiang L, Wang X, Li W, Zhuang H, Zhang X, Chen H, Li S, Yang Y, Lu Y, Wang J, Zhu R, Zhang L, Sui S, Tan N, Zhao B, Zhang J, Li L, Feng D (2016) FUNDC1 regulates mitochondrial dynamics at the ER-mitochondrial contact site under hypoxic conditions. Embo j 35(13):1368–1384. https://doi.org/10.15252/embj.201593102
Ney PA (2015) Mitochondrial autophagy: Origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta 1853 (10 Pt B):2775-2783. doi:https://doi.org/10.1016/j.bbamcr.2015.02.022
Ferrer I (2006) Apoptosis: future targets for neuroprotective strategies. Cerebrovasc Dis 21(Suppl 2):9–20. https://doi.org/10.1159/000091699
Wei H, Li Y, Han S, Liu S, Zhang N, Zhao L, Li S, Li J (2016) cPKCγ-Modulated Autophagy in Neurons Alleviates Ischemic Injury in Brain of Mice with Ischemic Stroke Through Akt-mTOR Pathway. Transl Stroke Res 7(6):497–511. https://doi.org/10.1007/s12975-016-0484-4
Galluzzi L, Kepp O, Kroemer G (2012) Mitochondria: master regulators of danger signalling. Nature Reviews Molecular Cell Biology 13(12):780–788. https://doi.org/10.1038/nrm3479
Hertz L (2008) Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 55(3):289–309. https://doi.org/10.1016/j.neuropharm.2008.05.023
Chaudhari N, Talwar P, Parimisetty A, Lefebvre d’Hellencourt C, Ravanan P (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8:213. https://doi.org/10.3389/fncel.2014.00213
Ruiz A, Matute C, Alberdi E (2009) Endoplasmic reticulum Ca(2+) release through ryanodine and IP(3) receptors contributes to neuronal excitotoxicity. Cell Calcium 46(4):273–281. https://doi.org/10.1016/j.ceca.2009.08.005
Webster KA (2012) Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol 8(6):863–884. https://doi.org/10.2217/fca.12.58
Liu Z, Chopp M (2016) Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol 144:103–120. https://doi.org/10.1016/j.pneurobio.2015.09.008
Benarroch EE (2005) Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc 80(10):1326–1338. https://doi.org/10.4065/80.10.1326
Schousboe A, Westergaard N, Sonnewald U, Petersen SB, Yu AC, Hertz L (1992) Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Prog Brain Res 94:199–211. https://doi.org/10.1016/s0079-6123(08)61751-3
Takano T, Oberheim N, Cotrina ML, Nedergaard M (2009) Astrocytes and ischemic injury. Stroke 40(3 Suppl):S8-12. https://doi.org/10.1161/strokeaha.108.533166
Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32(18):6391–6410. https://doi.org/10.1523/jneurosci.6221-11.2012
Rouach N, Glowinski J, Giaume C (2000) Activity-dependent neuronal control of gap-junctional communication in astrocytes. J Cell Biol 149(7):1513–1526. https://doi.org/10.1083/jcb.149.7.1513
Rose CR, Ransom BR (1997) Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia 20(4):299–307. https://doi.org/10.1002/(sici)1098-1136(199708)20:4%3c299::aid-glia3%3e3.0.co;2-1
Dong QP, He JQ, Chai Z (2013) Astrocytic Ca(2+) waves mediate activation of extrasynaptic NMDA receptors in hippocampal neurons to aggravate brain damage during ischemia. Neurobiol Dis 58:68–75. https://doi.org/10.1016/j.nbd.2013.05.005
Martínez AD, Sáez JC (2000) Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Brain Res Rev 32(1):250–258. https://doi.org/10.1016/s0165-0173(99)00086-7
Miao Y, Qiu Y, Lin Y, Miao Z, Zhang J, Lu X (2011) Protection by pyruvate against glutamate neurotoxicity is mediated by astrocytes through a glutathione-dependent mechanism. Mol Biol Rep 38(5):3235–3242. https://doi.org/10.1007/s11033-010-9998-0
Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG (2010) Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 58(9):1042–1049. https://doi.org/10.1002/glia.20985
Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG (2007) Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci 27(16):4253–4260. https://doi.org/10.1523/jneurosci.0211-07.2007
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555. https://doi.org/10.1038/nature18928
An J, Haile WB, Wu F, Torre E, Yepes M (2014) Tissue-type plasminogen activator mediates neuroglial coupling in the central nervous system. Neuroscience 257:41–48. https://doi.org/10.1016/j.neuroscience.2013.10.060
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27(11):1766–1791. https://doi.org/10.1038/sj.jcbfm.9600521
Amato S, Man HY (2011) Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle 10(20):3452–3460. https://doi.org/10.4161/cc.10.20.17953
Benakis C, Garcia-Bonilla L, Iadecola C, Anrather J (2014) The role of microglia and myeloid immune cells in acute cerebral ischemia. Front Cell Neurosci 8:461. https://doi.org/10.3389/fncel.2014.00461
Ye J, Jiang Z, Chen X, Liu M, Li J, Liu N (2016) Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp Cell Res 340(2):315–326. https://doi.org/10.1016/j.yexcr.2015.10.026
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35. https://doi.org/10.1038/nri978
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45. https://doi.org/10.1186/1742-2094-5-45
Kapellos TS, Taylor L, Lee H, Cowley SA, James WS, Iqbal AJ, Greaves DR (2016) A novel real time imaging platform to quantify macrophage phagocytosis. Biochem Pharmacol 116:107–119. https://doi.org/10.1016/j.bcp.2016.07.011
Bordt EA, Polster BM (2014) NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med 76:34–46. https://doi.org/10.1016/j.freeradbiomed.2014.07.033
Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA (2014) Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron 82(1):195–207. https://doi.org/10.1016/j.neuron.2014.01.043
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. https://doi.org/10.1038/nrn2038
Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y (2018) Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother 105:518–525. https://doi.org/10.1016/j.biopha.2018.05.143
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Lei Q, Tan J, Yi S, Wu N, Wang Y, Wu H (2018) Mitochonic acid 5 activates the MAPK-ERK-yap signaling pathways to protect mouse microglial BV-2 cells against TNFα-induced apoptosis via increased Bnip3-related mitophagy. Cell Mol Biol Lett 23:14. https://doi.org/10.1186/s11658-018-0081-5
Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M (2016) Parkinson’s Disease-Related Proteins PINK1 and Parkin Repress Mitochondrial Antigen Presentation. Cell 166(2):314–327. https://doi.org/10.1016/j.cell.2016.05.039
Strazielle N, Ghersi-Egea JF (2013) Physiology of blood-brain interfaces in relation to brain disposition of small compounds and macromolecules. Mol Pharm 10(5):1473–1491. https://doi.org/10.1021/mp300518e
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V (2007) Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 6(3):258–268. https://doi.org/10.1016/s1474-4422(07)70055-8
Rayasam A, Hsu M, Kijak JA, Kissel L, Hernandez G, Sandor M, Fabry Z (2018) Immune responses in stroke: how the immune system contributes to damage and healing after stroke and how this knowledge could be translated to better cures? Immunology 154(3):363–376. https://doi.org/10.1111/imm.12918
Moroni F (2008) Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol 8(1):96–103. https://doi.org/10.1016/j.coph.2007.10.005
Zhang Y, Park TS, Gidday JM (2007) Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol 292(6):H2573-2581. https://doi.org/10.1152/ajpheart.01098.2006
Dong H, Zhou W, Xin J, Shi H, Yao X, He Z, Wang Z (2019) Salvinorin A moderates postischemic brain injury by preserving endothelial mitochondrial function via AMPK/Mfn2 activation. Exp Neurol 322:113045. https://doi.org/10.1016/j.expneurol.2019.113045
Chen T, Dai SH, Li X, Luo P, Zhu J, Wang YH, Fei Z, Jiang XF (2018) Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol 14:229–236. https://doi.org/10.1016/j.redox.2017.09.016
Ruan L, Wang B, ZhuGe Q, Jin K (2015) Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res 1623:166–173. https://doi.org/10.1016/j.brainres.2015.02.042
Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436(7048):193–200. https://doi.org/10.1038/nature03875
Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A (2013) Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One 8(1):e55039. https://doi.org/10.1371/journal.pone.0055039
Fujioka T, Kaneko N, Ajioka I, Nakaguchi K, Omata T, Ohba H, Fässler R, García-Verdugo JM, Sekiguchi K, Matsukawa N, Sawamoto K (2017) β1 integrin signaling promotes neuronal migration along vascular scaffolds in the post-stroke brain. EBioMedicine 16:195–203. https://doi.org/10.1016/j.ebiom.2017.01.005
Semenza GL (2009) Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 24:97–106. https://doi.org/10.1152/physiol.00045.2008
Ziello JE, Jovin IS, Huang Y (2007) Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 80(2):51–60
Malhotra D, Portales-Casamar E, Singh A, Srivastava S, Arenillas D, Happel C, Shyr C, Wakabayashi N, Kensler TW, Wasserman WW, Biswal S (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res 38(17):5718–5734. https://doi.org/10.1093/nar/gkq212
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13(1):76–86. https://doi.org/10.1101/gad.13.1.76
Dinkova-Kostova AT, Abramov AY (2015) The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med 88(Pt B):179–188. https://doi.org/10.1016/j.freeradbiomed.2015.04.036
Holmström KM, Baird L, Zhang Y, Hargreaves I, Chalasani A, Land JM, Stanyer L, Yamamoto M, Dinkova-Kostova AT, Abramov AY (2013) Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biology Open 2(8):761. https://doi.org/10.1242/bio.20134853
Itoh K, Ye P, Matsumiya T, Tanji K, Ozaki T (2015) Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. Journal of Clinical Biochemistry and Nutrition 56(2):91–97. https://doi.org/10.3164/jcbn.14-134
Gibson CJ, Davids MS (2015) BCL-2 Antagonism to Target the Intrinsic Mitochondrial Pathway of Apoptosis. Clin Cancer Res 21(22):5021–5029. https://doi.org/10.1158/1078-0432.Ccr-15-0364
Green DR (2000) Apoptotic pathways: paper wraps stone blunts scissors. Cell 102(1):1–4. https://doi.org/10.1016/s0092-8674(00)00003-9
Yang Y, Yu Y, Wang J, Li Y, Li Y, Wei J, Zheng T, Jin M, Sun Z (2017) Silica nanoparticles induced intrinsic apoptosis in neuroblastoma SH-SY5Y cells via CytC/Apaf-1 pathway. Environ Toxicol Pharmacol 52:161–169. https://doi.org/10.1016/j.etap.2017.01.010
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275(5303):1132–1136. https://doi.org/10.1126/science.275.5303.1132
Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91(5):627–637. https://doi.org/10.1016/s0092-8674(00)80450-x
Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A 95(9):4997–5002. https://doi.org/10.1073/pnas.95.9.4997
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94(4):491–501. https://doi.org/10.1016/s0092-8674(00)81590-1
Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ (2000) tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev 14(16):2060–2071
Zhou J, Du T, Li B, Rong Y, Verkhratsky A, Peng L (2015) Crosstalk Between MAPK/ERK and PI3K/AKT Signal Pathways During Brain Ischemia/Reperfusion. ASN Neuro 7 (5). doi:https://doi.org/10.1177/1759091415602463
Kawano T, Morioka M, Yano S, Hamada J, Ushio Y, Miyamoto E, Fukunaga K (2002) Decreased akt activity is associated with activation of forkhead transcription factor after transient forebrain ischemia in gerbil hippocampus. J Cereb Blood Flow Metab 22(8):926–934. https://doi.org/10.1097/00004647-200208000-00004
Xing XS, Liu F, He ZY (2015) Akt regulates β-catenin in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep 11(4):3122–3128. https://doi.org/10.3892/mmr.2014.3000
Ban K, Peng Z, Kozar RA (2013) Inhibition of ERK1/2 worsens intestinal ischemia/reperfusion injury. PLoS One 8(9):e76790. https://doi.org/10.1371/journal.pone.0076790
Dhandapani KM, Brann DW (2003) Transforming growth factor-beta: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys 39(1):13–22. https://doi.org/10.1385/cbb:39:1:13
Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D (2003) Transforming growth factor-beta and ischemic brain injury. Cell Mol Neurobiol 23(4–5):539–550. https://doi.org/10.1023/a:1025072013107
Ridder DA, Schwaninger M (2009) NF-kappaB signaling in cerebral ischemia. Neuroscience 158(3):995–1006. https://doi.org/10.1016/j.neuroscience.2008.07.007
Inta I, Paxian S, Maegele I, Zhang W, Pizzi M, Spano P, Sarnico I, Muhammad S, Herrmann O, Inta D, Baumann B, Liou HC, Schmid RM, Schwaninger M (2006) Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J Neurosci 26(50):12896–12903. https://doi.org/10.1523/jneurosci.3670-06.2006
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453(7196):807–811. https://doi.org/10.1038/nature06905
Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Köhrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M (2005) IKK mediates ischemia-induced neuronal death. Nat Med 11(12):1322–1329. https://doi.org/10.1038/nm1323
Kim GS, Jung JE, Narasimhan P, Sakata H, Yoshioka H, Song YS, Okami N, Chan PH (2012) Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J Cereb Blood Flow Metab 32(4):720–730. https://doi.org/10.1038/jcbfm.2011.176
Mandal T, Bhowmik A, Chatterjee A, Chatterjee U, Chatterjee S, Ghosh MK (2014) Reduced phosphorylation of Stat3 at Ser-727 mediated by casein kinase 2 - protein phosphatase 2A enhances Stat3 Tyr-705 induced tumorigenic potential of glioma cells. Cell Signal 26(8):1725–1734. https://doi.org/10.1016/j.cellsig.2014.04.003
Jung JE, Kim GS, Chan PH (2011) Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke 42(12):3574–3579. https://doi.org/10.1161/strokeaha.111.626648
Chao CC, Ma YL, Lee EH (2011) Brain-derived neurotrophic factor enhances Bcl-xL expression through protein kinase casein kinase 2-activated and nuclear factor kappa B-mediated pathway in rat hippocampus. Brain Pathol 21(2):150–162. https://doi.org/10.1111/j.1750-3639.2010.00431.x
Afonyushkin T, Oskolkova OV, Binder BR, Bochkov VN (2011) Involvement of CK2 in activation of electrophilic genes in endothelial cells by oxidized phospholipids. J Lipid Res 52(1):98–103. https://doi.org/10.1194/jlr.M009480
Baltan S, Bastian C, Quinn J, Aquila D, McCray A, Brunet S (2018) CK2 inhibition protects white matter from ischemic injury. Neurosci Lett 687:37–42. https://doi.org/10.1016/j.neulet.2018.08.021
Bastian C, Quinn J, Tripathi A, Aquila D, McCray A, Dutta R, Baltan S, Brunet S (2019) CK2 inhibition confers functional protection to young and aging axons against ischemia by differentially regulating the CDK5 and AKT signaling pathways. Neurobiol Dis 126:47–61. https://doi.org/10.1016/j.nbd.2018.05.011
Meyer DA, Torres-Altoro MI, Tan Z, Tozzi A, Di Filippo M, DiNapoli V, Plattner F, Kansy JW, Benkovic SA, Huber JD, Miller DB, Greengard P, Calabresi P, Rosen CL, Bibb JA (2014) Ischemic stroke injury is mediated by aberrant Cdk5. J Neurosci 34(24):8259–8267. https://doi.org/10.1523/jneurosci.4368-13.2014
Tan X, Chen Y, Li J, Li X, Miao Z, Xin N, Zhu J, Ge W, Feng Y, Xu X (2015) The inhibition of Cdk5 activity after hypoxia/ischemia injury reduces infarct size and promotes functional recovery in neonatal rats. Neuroscience 290:552–560. https://doi.org/10.1016/j.neuroscience.2015.01.054
Wacquier B, Combettes L, Dupont G (2019) Cytoplasmic and Mitochondrial Calcium Signaling: A Two-Way Relationship. Cold Spring Harb Perspect Biol 11 (10). doi:https://doi.org/10.1101/cshperspect.a035139
Bhosale G, Sharpe JA, Sundier SY, Duchen MR (2015) Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann N Y Acad Sci 1350(1):107–116. https://doi.org/10.1111/nyas.12885
Kowaltowski AJ, Menezes-Filho SL, Assali EA, Gonçalves IG, Cabral-Costa JV, Abreu P, Miller N, Nolasco P, Laurindo FRM, Bruni-Cardoso A, Shirihai OS (2019) Mitochondrial morphology regulates organellar Ca(2+) uptake and changes cellular Ca(2+) homeostasis. Faseb j 33(12):13176–13188. https://doi.org/10.1096/fj.201901136R
Stys PK, Waxman SG, Ransom BR (1992) Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci 12(2):430–439. https://doi.org/10.1523/jneurosci.12-02-00430.1992
Imahashi K, Kusuoka H, Hashimoto K, Yoshioka J, Yamaguchi H, Nishimura T (1999) Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ Res 84(12):1401–1406. https://doi.org/10.1161/01.res.84.12.1401
Brustovetsky T, Bolshakov A, Brustovetsky N (2010) Calpain activation and Na+/Ca2+ exchanger degradation occur downstream of calcium deregulation in hippocampal neurons exposed to excitotoxic glutamate. J Neurosci Res 88(6):1317–1328. https://doi.org/10.1002/jnr.22295
Bano D, Nicotera P (2007) Ca2+ signals and neuronal death in brain ischemia. Stroke 38(2 Suppl):674–676. https://doi.org/10.1161/01.Str.0000256294.46009.29
Polster BM (2013) AIF, reactive oxygen species, and neurodegeneration: a “complex” problem. Neurochem Int 62(5):695–702. https://doi.org/10.1016/j.neuint.2012.12.002
Bolaños JP, Almeida A (1999) Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta 1411(2–3):415–436. https://doi.org/10.1016/s0005-2728(99)00030-4
Forman LJ, Liu P, Nagele RG, Yin K, Wong PY (1998) Augmentation of nitric oxide, superoxide, and peroxynitrite production during cerebral ischemia and reperfusion in the rat. Neurochem Res 23(2):141–148. https://doi.org/10.1023/a:1022468522564
Narendra D, Walker JE, Youle R (2012) Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol 4 (11). doi:https://doi.org/10.1101/cshperspect.a011338
Di Sante G, Pestell TG, Casimiro MC, Bisetto S, Powell MJ, Lisanti MP, Cordon-Cardo C, Castillo-Martin M, Bonal DM, Debattisti V, Chen K, Wang L, He X, McBurney MW, Pestell RG (2015) Loss of Sirt1 promotes prostatic intraepithelial neoplasia, reduces mitophagy, and delays PARK2 translocation to mitochondria. Am J Pathol 185(1):266–279. https://doi.org/10.1016/j.ajpath.2014.09.014
Elmore SP, Qian T, Grissom SF, Lemasters JJ (2001) The mitochondrial permeability transition initiates autophagy in rat hepatocytes. Faseb j 15(12):2286–2287. https://doi.org/10.1096/fj.01-0206fje
Solesio ME, Saez-Atienzar S, Jordan J, Galindo MF (2013) 3-Nitropropionic acid induces autophagy by forming mitochondrial permeability transition pores rather than activating the mitochondrial fission pathway. Br J Pharmacol 168(1):63–75. https://doi.org/10.1111/j.1476-5381.2012.01994.x
Cui T, Fan C, Gu L, Gao H, Liu Q, Zhang T, Qi Z, Zhao C, Zhao H, Cai Q, Yang H (2011) Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Res 1394:1–13. https://doi.org/10.1016/j.brainres.2011.01.035
Gustafsson AB (2011) Bnip3 as a dual regulator of mitochondrial turnover and cell death in the myocardium. Pediatr Cardiol 32(3):267–274. https://doi.org/10.1007/s00246-010-9876-5
Zuo W, Yang PF, Chen J, Zhang Z, Chen NH (2016) Drp-1, a potential therapeutic target for brain ischaemic stroke. Br J Pharmacol 173(10):1665–1677. https://doi.org/10.1111/bph.13468
Zuo W, Zhang S, Xia CY, Guo XF, He WB, Chen NH (2014) Mitochondria autophagy is induced after hypoxic/ischemic stress in a Drp1 dependent manner: the role of inhibition of Drp1 in ischemic brain damage. Neuropharmacology 86:103–115. https://doi.org/10.1016/j.neuropharm.2014.07.002
Yu W, Sun Y, Guo S, Lu B (2011) The PINK1/Parkin pathway regulates mitochondrial dynamics and function in mammalian hippocampal and dopaminergic neurons. Hum Mol Genet 20(16):3227–3240. https://doi.org/10.1093/hmg/ddr235
Feng J, Chen X, Guan B, Li C, Qiu J, Shen J (2018) Inhibition of Peroxynitrite-Induced Mitophagy Activation Attenuates Cerebral Ischemia-Reperfusion Injury. Mol Neurobiol 55(8):6369–6386. https://doi.org/10.1007/s12035-017-0859-x
Wang W, Karamanlidis G, Tian R (2016) Novel targets for mitochondrial medicine. Sci Transl Med 8 (326):326rv323. doi:https://doi.org/10.1126/scitranslmed.aac7410
Cui M, Ding H, Chen F, Zhao Y, Yang Q, Dong Q (2016) Mdivi-1 Protects Against Ischemic Brain Injury via Elevating Extracellular Adenosine in a cAMP/CREB-CD39-Dependent Manner. Mol Neurobiol 53(1):240–253. https://doi.org/10.1007/s12035-014-9002-4
Gao D, Zhang L, Dhillon R, Hong TT, Shaw RM, Zhu J (2013) Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One 8(4):e60967. https://doi.org/10.1371/journal.pone.0060967
Qi X, Qvit N, Su YC, Mochly-Rosen D (2013) A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci 126(Pt 3):789–802. https://doi.org/10.1242/jcs.114439
Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K (2009) Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol 11(8):958–966. https://doi.org/10.1038/ncb1907
Elachouri G, Vidoni S, Zanna C, Pattyn A, Boukhaddaoui H, Gaget K, Yu-Wai-Man P, Gasparre G, Sarzi E, Delettre C, Olichon A, Loiseau D, Reynier P, Chinnery PF, Rotig A, Carelli V, Hamel CP, Rugolo M, Lenaers G (2011) OPA1 links human mitochondrial genome maintenance to mtDNA replication and distribution. Genome Res 21(1):12–20. https://doi.org/10.1101/gr.108696.110
Szabo A, Sumegi K, Fekete K, Hocsak E, Debreceni B, Setalo G Jr, Kovacs K, Deres L, Kengyel A, Kovacs D, Mandl J, Nyitrai M, Febbraio MA, Gallyas F Jr, Sumegi B (2018) Activation of mitochondrial fusion provides a new treatment for mitochondria-related diseases. Biochem Pharmacol 150:86–96. https://doi.org/10.1016/j.bcp.2018.01.038
Smith MA, Schnellmann RG (2012) Calpains, mitochondria, and apoptosis. Cardiovasc Res 96(1):32–37. https://doi.org/10.1093/cvr/cvs163
Arrington DD, Van Vleet TR, Schnellmann RG (2006) Calpain 10: a mitochondrial calpain and its role in calcium-induced mitochondrial dysfunction. Am J Physiol Cell Physiol 291(6):C1159-1171. https://doi.org/10.1152/ajpcell.00207.2006
Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8(10):1124–1132. https://doi.org/10.1038/ncb1482
Ono Y, Saido TC, Sorimachi H (2016) Calpain research for drug discovery: challenges and potential. Nat Rev Drug Discov 15(12):854–876. https://doi.org/10.1038/nrd.2016.212
Yokota M, Tani E, Tsubuki S, Yamaura I, Nakagaki I, Hori S, Saido TC (1999) Calpain inhibitor entrapped in liposome rescues ischemic neuronal damage. Brain Res 819(1–2):8–14. https://doi.org/10.1016/s0006-8993(98)01334-1
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14(8):1505–1517. https://doi.org/10.1089/ars.2010.3576
Coucha M, Li W, Hafez S, Abdelsaid M, Johnson MH, Fagan SC, Ergul A (2015) SOD1 overexpression prevents acute hyperglycemia-induced cerebral myogenic dysfunction: relevance to contralateral hemisphere and stroke outcomes. Am J Physiol Heart Circ Physiol 308(5):H456-466. https://doi.org/10.1152/ajpheart.00321.2014
Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D (2003) On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol 140(3):445–460. https://doi.org/10.1038/sj.bjp.0705430
Wen Y, Li W, Poteet EC, Xie L, Tan C, Yan LJ, Ju X, Liu R, Qian H, Marvin MA, Goldberg MS, She H, Mao Z, Simpkins JW, Yang SH (2011) Alternative mitochondrial electron transfer as a novel strategy for neuroprotection. J Biol Chem 286(18):16504–16515. https://doi.org/10.1074/jbc.M110.208447
Huang S, Du F, Shih YY, Shen Q, Gonzalez-Lima F, Duong TQ (2013) Methylene blue potentiates stimulus-evoked fMRI responses and cerebral oxygen consumption during normoxia and hypoxia. Neuroimage 72:237–242. https://doi.org/10.1016/j.neuroimage.2013.01.027
Poteet E, Winters A, Yan LJ, Shufelt K, Green KN, Simpkins JW, Wen Y, Yang SH (2012) Neuroprotective actions of methylene blue and its derivatives. PLoS One 7(10):e48279. https://doi.org/10.1371/journal.pone.0048279
Sheu SS, Nauduri D, Anders MW (2006) Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta 1762(2):256–265. https://doi.org/10.1016/j.bbadis.2005.10.007
McManus MJ, Murphy MP, Franklin JL (2014) Mitochondria-derived reactive oxygen species mediate caspase-dependent and -independent neuronal deaths. Mol Cell Neurosci 63:13–23. https://doi.org/10.1016/j.mcn.2014.09.002
Smith RA, Porteous CM, Gane AM, Murphy MP (2003) Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A 100(9):5407–5412. https://doi.org/10.1073/pnas.0931245100
Skulachev VP, Anisimov VN, Antonenko YN, Bakeeva LE, Chernyak BV, Erichev VP, Filenko OF, Kalinina NI, Kapelko VI, Kolosova NG, Kopnin BP, Korshunova GA, Lichinitser MR, Obukhova LA, Pasyukova EG, Pisarenko OI, Roginsky VA, Ruuge EK, Senin II, Severina II, Skulachev MV, Spivak IM, Tashlitsky VN, Tkachuk VA, Vyssokikh MY, Yaguzhinsky LS, Zorov DB (2009) An attempt to prevent senescence: a mitochondrial approach. Biochim Biophys Acta 1787(5):437–461. https://doi.org/10.1016/j.bbabio.2008.12.008
Hu Q, Ren J, Li G, Wu J, Wu X, Wang G, Gu G, Ren H, Hong Z, Li J (2018) The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis 9(3):403. https://doi.org/10.1038/s41419-018-0436-x
Zhao Y, Lee JH, Chen D, Gu X, Caslin A, Li J, Yu SP, Wei L (2017) DL-3-n-butylphthalide induced neuroprotection, regenerative repair, functional recovery and psychological benefits following traumatic brain injury in mice. Neurochem Int 111:82–92. https://doi.org/10.1016/j.neuint.2017.03.017
Wen XR, Tang M, Qi DS, Huang XJ, Liu HZ, Zhang F, Wu J, Wang YW, Zhang XB, Guo JQ, Wang SL, Liu Y, Wang YL, Song YJ (2016) Butylphthalide Suppresses Neuronal Cells Apoptosis and Inhibits JNK-Caspase3 Signaling Pathway After Brain Ischemia /Reperfusion in Rats. Cell Mol Neurobiol 36(7):1087–1095. https://doi.org/10.1007/s10571-015-0302-7
Li J, Li Y, Ogle M, Zhou X, Song M, Yu SP, Wei L (2010) DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 1359:216–226. https://doi.org/10.1016/j.brainres.2010.08.061
Chen N, Zhou Z, Li J, Li B, Feng J, He D, Luo Y, Zheng X, Luo J, Zhang J (2018) 3-n-butylphthalide exerts neuroprotective effects by enhancing anti-oxidation and attenuating mitochondrial dysfunction in an in vitro model of ischemic stroke. Drug Des Devel Ther 12:4261–4271. https://doi.org/10.2147/dddt.S189472
Yin W, Lan L, Huang Z, Ji J, Fang J, Wang X, Ji H, Peng S, Xu J, Zhang Y (2016) Discovery of a ring-opened derivative of 3-n-butylphthalide bearing NO/H2S-donating moieties as a potential anti-ischemic stroke agent. Eur J Med Chem 115:369–380. https://doi.org/10.1016/j.ejmech.2016.03.044
Zhang P, Guo ZF, Xu YM, Li YS, Song JG (2016) N-Butylphthalide (NBP) ameliorated cerebral ischemia reperfusion-induced brain injury via HGF-regulated TLR4/NF-κB signaling pathway. Biomed Pharmacother 83:658–666. https://doi.org/10.1016/j.biopha.2016.07.040
Yang CS, Guo A, Li Y, Shi K, Shi FD, Li M (2019) Dl-3-n-butylphthalide Reduces Neurovascular Inflammation and Ischemic Brain Injury in Mice. Aging Dis 10(5):964–976. https://doi.org/10.14336/ad.2019.0608
Bazan NG (2018) Docosanoids and elovanoids from omega-3 fatty acids are pro-homeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol Aspects Med 64:18–33. https://doi.org/10.1016/j.mam.2018.09.003
Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG (2011) Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl Stroke Res 2(1):33–41. https://doi.org/10.1007/s12975-010-0046-0
Mayurasakorn K, Niatsetskaya ZV, Sosunov SA, Williams JJ, Zirpoli H, Vlasakov I, Deckelbaum RJ, Ten VS (2016) DHA but Not EPA Emulsions Preserve Neurological and Mitochondrial Function after Brain Hypoxia-Ischemia in Neonatal Mice. PLoS One 11(8):e0160870. https://doi.org/10.1371/journal.pone.0160870
Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D (2012) Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol 33(1):85–104. https://doi.org/10.1016/j.yfrne.2011.10.001
Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y (2007) Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115(5):e69-171. https://doi.org/10.1161/circulationaha.106.179918
Guo H, Liu M, Zhang L, Wang L, Hou W, Ma Y, Ma Y (2020) The critical period for neuroprotection by estrogen replacement therapy and the potential underlying mechanisms. Curr Neuropharmacol. https://doi.org/10.2174/1570159x18666200123165652
Rau SW, Dubal DB, Böttner M, Gerhold LM, Wise PM (2003) Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci 23(36):11420–11426. https://doi.org/10.1523/jneurosci.23-36-11420.2003
Wu TW, Wang JM, Chen S, Brinton RD (2005) 17Beta-estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135(1):59–72. https://doi.org/10.1016/j.neuroscience.2004.12.027
Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM (2001) Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A 98(4):1952–1957. https://doi.org/10.1073/pnas.041483198
Merchenthaler I, Dellovade TL, Shughrue PJ (2003) Neuroprotection by estrogen in animal models of global and focal ischemia. Ann N Y Acad Sci 1007:89–100. https://doi.org/10.1196/annals.1286.009
Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schütz G, Schwaninger M (2010) Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. J Cereb Blood Flow Metab 30(5):935–942. https://doi.org/10.1038/jcbfm.2009.258
Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM (2010) Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci 32(12):1995–2002. https://doi.org/10.1111/j.1460-9568.2010.07516.x
Brinton RD (2005) Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci 1052:57–74. https://doi.org/10.1196/annals.1347.005
Brinton RD (2008) The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci 31(10):529–537. https://doi.org/10.1016/j.tins.2008.07.003
Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH (2010) Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab 95(7 Suppl 1):s1–s66. https://doi.org/10.1210/jc.2009-2509
Lee JY, Xu K, Nguyen H, Guedes VA, Borlongan CV, Acosta SA (2017) Stem Cell-Induced Biobridges as Possible Tools to Aid Neuroreconstruction after CNS Injury. Front Cell Dev Biol 5:51. https://doi.org/10.3389/fcell.2017.00051
Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, Arai K, Rosell A, Lo EH (2018) Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 36(9):1404–1410. https://doi.org/10.1002/stem.2856
Lin HY, Liou CW, Chen SD, Hsu TY, Chuang JH, Wang PW, Huang ST, Tiao MM, Chen JB, Lin TK, Chuang YC (2015) Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 22:31–44. https://doi.org/10.1016/j.mito.2015.02.006
Torralba D, Baixauli F, Sánchez-Madrid F (2016) Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front Cell Dev Biol 4:107. https://doi.org/10.3389/fcell.2016.00107
Berridge MV, McConnell MJ, Grasso C, Bajzikova M, Kovarova J, Neuzil J (2016) Horizontal transfer of mitochondria between mammalian cells: beyond co-culture approaches. Curr Opin Genet Dev 38:75–82. https://doi.org/10.1016/j.gde.2016.04.003
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, Yan C (2014) Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 92:10–18. https://doi.org/10.1016/j.mvr.2014.01.008
Mahrouf-Yorgov M, Augeul L, Da Silva CC, Jourdan M, Rigolet M, Manin S, Ferrera R, Ovize M, Henry A, Guguin A, Meningaud JP, Dubois-Randé JL, Motterlini R, Foresti R, Rodriguez AM (2017) Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ 24(7):1224–1238. https://doi.org/10.1038/cdd.2017.51
Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH (2018) Extracellular Mitochondria for Therapy and Diagnosis in Acute Central Nervous System Injury. JAMA Neurol 75(1):119–122. https://doi.org/10.1001/jamaneurol.2017.3475
Wang X, Gerdes HH (2015) Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ 22(7):1181–1191. https://doi.org/10.1038/cdd.2014.211
He Z, Ning N, Zhou Q, Khoshnam SE, Farzaneh M (2020) Mitochondria as a therapeutic target for ischemic stroke. Free Radic Biol Med 146:45–58. https://doi.org/10.1016/j.freeradbiomed.2019.11.005
Nakamura Y, Lo EH, Hayakawa K (2020) Placental Mitochondria Therapy for Cerebral Ischemia-Reperfusion Injury in Mice. Stroke 51(10):3142–3146. https://doi.org/10.1161/strokeaha.120.030152
Li Y, Sun J, Wu R, Bai J, Hou Y, Zeng Y, Zhang Y, Wang X, Wang Z, Meng X (2020) Mitochondrial MPTP: A Novel Target of Ethnomedicine for Stroke Treatment by Apoptosis Inhibition. Front Pharmacol 11:352. https://doi.org/10.3389/fphar.2020.00352
Zhang F, Lin YA, Kannan S, Kannan RM (2016) Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release 240:212–226. https://doi.org/10.1016/j.jconrel.2015.12.013
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (General Program 81971115, and 82071306), and the Beijing Key Laboratory (BZ0250).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, J., Jin, H., Nan, D. et al. New insights into targeting mitochondria in ischemic injury. Apoptosis 26, 163–183 (2021). https://doi.org/10.1007/s10495-021-01661-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01661-5