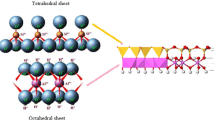
Abstract
The present study was aimed at the preparation and characterization of tailor made hybrid materials, whose peculiar hosting capability could be exploited in biotechnological applications. With this purpose, the modification of K10 montmorillonite by intercalation of Tween 20 surfactant, was accomplished. The influence of two internal parameters, namely pH and surfactant/clay ratio, on the surfactant uptake ability by clay was investigated. The adsorption mechanism was elucidated on the basis of complementary kinetic and equilibrium studies and, then, corroborated by the useful information provided by the FT-IR, TGA and XRD characterization. The gathered results allow to draw the conclusion that the whole surfactant uptake is the result of two contributions: a site-limiting component, governed by negative cooperative interactions, which takes into account for the Tween 20 adsorption onto the pristine clay, and a non-specific linear partitioning component, related to the adsorption of the surfactant onto the in situ prepared organo-clay. Moreover, at strongly acidic pH, a mechanism consisting of two-steps pathways involving two non-energetically equivalent binding sites of the clay surfaces, was proposed, while, on increasing the pH, the clay interlayer becomes the sole available site for the surfactant uptake. In the light of the interesting results obtained, among the plethora of potential biotechnological applications, the present paper suggests the exploitation of the prepared organo-clays to improve the performance of either hydrophilic or hydrophobic drug carriers systems.












Similar content being viewed by others
References
Arellano-Cardenas, S., Gallardo-Velazquez, T., Poumian-Gamboa, G.V., Osorio-Revilla, G., Lopez-Cortez, S., Rivera-Espinoza, Y.: Sorption of naringin from aqueous solution by modified clay. Clays Clay Miner. 60, 153–161 (2012)
Bordbar, A.K., Saboury, A.A., Moosavi-Movahedi, A.: The shapes of Scatchard plots for systems with two sets of binding sites. Biochemistry 24, 172–173 (1996)
Bromberg, L., Straut, C.M., Centrone, A., Wilusz, E., Hatton, T.A.: Deconvolution procedure of the UV-vis spectra. A powerful tool for the estimation of the binding of a model drug to specific solubilisation loci of bio-compatible aqueous surfactant-forming micelle. ACS Appl. Mater. Interfaces. 3, 1479–1484 (2011)
Bujdák, J., Hackett, E., Giannelis, E.P.: Effect of layer charge on the intercalation of poly(ethylene oxide) in layered silicates: implications on nanocomposite polymer electrolytes. Chem. Mater. 12, 2168–2174 (2000)
Caglioti, G., Paoletti, A., Ricci, F.P.: Choice of collimators for a crystal spectrometer for neutron difiraction. Nucl. Instrum. 3, 223–228 (1958)
Calabrese, I., Gelardi, G., Merli, M., Rytwo, G., Sciascia, L., Turco Liveri, ML.: New tailor-made bio-organoclays for the remediation of olive mill waste water. In: IOP Conferences Series: Materials Science and Engineering, vol. 47, p. 012040 (2013a)
Calabrese, I., Cavallaro, G., Scialabba, C., Licciardi, M., Merli, M., Sciascia, L., Turco Liveri, M.L.: Montmorillonite nanodevices for the colon metronidazole delivery. Int. J. Pharm. 457, 224–236 (2013)
Calabrese, I., Merli, M., Turco Liveri, M.L.: Deconvolution procedure of the UV-vis spectra. A powerful tool for the estimation of the binding of a model drug to specific solubilisation loci of bio-compatible aqueous surfactant-forming micelle. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 142, 150–158 (2015)
Cavallaro, G., Lazzara, G., Milioto, S.: Dispersions of nanoclays of different shapes into aqueous and solid biopolymeric matrices. Extended physicochemical study. Langmuir 27, 1158–1167 (2011)
Cavallaro, G., Lazzara, G., Milioto, S., Palmisano, G., Parisi, F.: Halloysite nanotube with fluorinated lumen: non-foaming nanocontainer for storage and controlled release of oxygen in aqueous media. J. Colloid Interface Sci. 417, 66–71 (2014a)
Cavallaro, G., Lazzara, G., Milioto, S., Parisi, F., Sanzillo, V.: Modified halloysite nanotubes: nanoarchitectures for enhancing the capture of oils from vapor and liquid phases. ACS Appl. Mater. Interfaces. 6, 606–612 (2014b)
Cavallaro, G., Lazzara, G., Milioto, S.: Exploiting the colloidal stability and solubilization ability of clay nanotubes/ionic surfactant hybrid nanomaterials. J. Phys. Chem. C 116, 21932–21938 (2012)
Chiron, N., Guilet, R., Deydier, E.: Adsorption of Cu(II) and Pb(II) onto a grafted silica: isotherms and kinetic models. Water Res. 37, 3079–3086 (2003)
Costa, P., Sousa Lobo, J.M.: Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 13, 123–133 (2001)
Cui, L., Tarte, N.H., Woo, S.I.: Effects of modified clay on the morphology and properties of PMMA/clay nanocomposites synthesized by in situ polymerization. Macromolecules 41, 4268–4274 (2008)
del Hoyo, C., Dorado, C., Rodríguez-Cruz, M.S., Sánchez-Martín, M.J.: Physico-chemical study of selected surfactant–clay mineral systems. J. Therm. Anal. Calorim. 94, 227–234 (2008)
Deng, Y., Dixon, J.B., White, G.: Norman Intercalation and surface modification of smectite by two non-ionic surfactants. Clays Clay Miner. 51, 150–161 (2003)
Deng, Y., Dixon, J.B., White, N.: Bonding mechanisms and conformation of poly(ethylene oxide)-based surfactants in interlayer of smectite. Colloid Polym. Sci. 284, 347–356 (2006)
Ghiaci, M., Kalbasi, R.J., Abbaspour, A.: Adsorption isotherms of non-ionic surfactants on Na-bentonite (Iran) and evaluation of hermodynamic parameters. Colloids Surf. Physicochem. Eng. Asp. 297, 105–113 (2007)
Guégan, R.: Self-assembly of a non-ionic surfactant onto a clay mineral for the preparation of hybrid layered materials. Soft Matter 9, 10913–10920 (2013)
Guégan, R., Gautier, M., Bény, J., Muller, F.: Adsorption of a C10E3 non-ionic surfactant on a Ca-smectite. Clays Clay Miner. 57, 502–509 (2009)
Koh, S.M., Dixon, J.B.: Preparation and application of organo-minerals as sorbents of phenol, benzene and toluene. Appl. Clay Sci. 18, 111–122 (2001)
Lee, S.M., Tiwari, D.: Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: an overview. Appl. Clay Sci. 59, 84–102 (2012)
Lin, F.H., Lee, Y.H., Jian, C.H., Wong, J.M., Shieh, M.J., Wang, C.Y.: A study of purified montmorillonite intercalated with 5-fluorouracil as drug carrier. Biomaterials 23, 1981–1987 (2002)
Liu, T., Ping Lim, K., Chauhari Tjiu, W., Pramoda, K.P., Chen, Z.K.: Preparation and characterization of nylon 11/organoclay nanocomposites. Polymer 44, 3529–3535 (2003)
Malik, U.R., Nasany, S.M., Subhani, M.S.: Sorptive potential of sunflower stem for Cr(III) ions from aqueous solutions and its kinetic and thermodynamic profile. Talanta 66, 166 (2005)
Merli, M., Sciascia, L., Turco Liveri, M.L.: Regression diagnostics applied in kinetic data processing: outlier recognition and robust weighting procedures. Int. J. Chem. Kinet. 42, 587–607 (2010)
Mortland, M.M., Shaobai, S., Boyd, S.A.: Clay-organic complexes as adsorbents for phenol and chlorophenols. Clays Clay Miner. 34, 581–586 (1986)
Nunes, C.D., Vaz, P.D., Fernandes, A.C., Ferreira, P., Romão, C.C., Calhorda, M.J.: Loanding and delivery of setraline using inorganic micro and mesoperous materials. Eur. J. Pharm. Biopharm. 66, 357–365 (2007)
Onder, E., Sarier, N., Ukuser, G., Ozturk, M., Arat, R.: Ultrasound assisted solvent free intercalation of montmorillonite with PEG1000: a new type of organoclay with improved thermal properties. Thermochim. Acta 566, 24–35 (2013)
Qiu, H., Lv, L., Pan, B., Zhang, Q., Zhang, W., Zhang, Q.: Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A. 10, 716–724 (2009)
Ratanajiajaroen, P., Watthanaphanit, A., Tamura, H., Tokura, S., Rujiravanit, R.: Release characteristic and stability of curcumin incorporated in β-chitin non-woven fibrous sheet using Tween 20 as an emulsifier. Eur. Polym. J. 48, 512–523 (2012)
Ruiz-Hitzky, E., Aranda, P., Darder, M., Rytwo, G.: Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 20, 9306–9321 (2010)
Sarier, N., Onder, E., Ozay, S., Ozkilic, Y.: Preparation of phase change material–montmorillonite composites suitable for thermal energy storage. Thermochim. Acta 524, 39–46 (2011)
Sánchez-Martín, M.J., Dorado, M.C., del Hoyo, C., Rodríguez-Cruz, M.S.: Influence of clay mineral structure and surfactant nature on the adsorption capacity of surfactants by clays. J. Hazard. Mater. 150, 115–123 (2008)
Sciascia, L., Turco Liveri, M.L., Merli, M.: Kinetic and equilibrium studies for the adsorption of acid nucleic bases onto K10 montmorillonite. Appl. Clay Sci. 53, 657–668 (2011)
Shen, Y.-H.: Removal of phenol from water by adsorption–flocculation using organo bentonite. Water Res. 36, 1107–1114 (2002)
Shen, Y.-H.: Preparations of organobentonite using non-ionic surfactants. Chemosphere 44, 989–995 (2001)
Singh, T.S., Pant, K.K.: Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Sep. Purif. Technol. 36, 139 (2004)
Tyagi, B., Chudasama, V., Jasra, R.V.: Determination of structural modification in acid activated montmorillonite clay by FT-IR spectroscopy. Spectr. Acta Part A 64, 273 (2006)
Turco Liveri, M.L., Licciardi, M., Sciascia, L., Giammona, G., Cavallaro, G.: Peculiar mechanism of solubilization of a sparingly water soluble drug into polymeric micelles. Kinetics and equilibrium studies. J. Phys. Chem. B 116, 5037–5046 (2012)
Xie, Y., Yu, H., Wang, S., Xu, Z.: Improvement of antifouling characteristics in a bioreactor of polypropylene microporous membrane by the adsorption of Tween 20. J. Environ. Sci. China. 19, 1461–1465 (2007)
Xu, X.R., Li, X.Y.: Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere 78, 430–436 (2010)
Zhang, Q., Yang, C., Huang, W., Dang, Z., Shu, X.: Sorption of tylosin on clay minerals. Chemosphere 93, 2180–2186 (2013)
Acknowledgments
The authors gratefully acknowledge the financial support provided by the MIUR (PRIN grant 2010EARRRZ_003) and local founds from University of Palermo (ex 60 %).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Calabrese, I., Cavallaro, G., Lazzara, G. et al. Preparation and characterization of bio-organoclays using nonionic surfactant. Adsorption 22, 105–116 (2016). https://doi.org/10.1007/s10450-015-9697-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9697-1