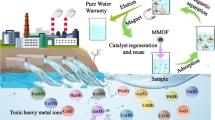
Abstract
Zeolite-based core–shell adsorbents are a promising new technology for removing toxic pollutants from aquatic environments. These adsorbents have a core of zeolite, a porous material with high adsorption capacity and selectivity. The outer shell of the adsorbent is made of another material, such as polymer, activated carbon, or metal. This structure provides several advantages, such as increased adsorption capacity, selectivity, and adsorbent stability. Zeolite-based core–shell adsorbents have shown great potential to remove toxic pollutants from aquatic environments, such as azo dye, heavy metals, and pharmaceuticals. However, there are still some challenges in the research and development of these adsorbents, such as developing simple and economical synthesis methods, improving adsorbent stability under different water conditions, and developing adsorbents that can remove multiple toxic pollutants simultaneously. Despite these challenges, zeolite-based core–shell adsorbents are a promising technology for removing toxic pollutants from aquatic environments. These adsorbents have the potential to provide an effective and economical solution to this serious environmental problem.




Copyright © 2016 The Authors, Published by Springer

Copyright © 2020 The Authors, Published by Willey


Copyright © 2022 The Authors, Published by American Institute of Chemical Engineers


Copyright © 2020 The Authors, Published by Heliyon
Similar content being viewed by others
References
Muruganandam M, Rajamanickam S, Sivarethinamohan S, Reddy MK, Velusamy P, Gomathi R, Ravindiran G, Gurugubelli TR, Munisamy SK (2023) Impact of climate change and anthropogenic activities on aquatic ecosystem – a review. Environ Res 238:117233. https://doi.org/10.1016/j.envres.2023.117233
Ertürk A, Ekdal A, Gürel M et al (2014) Evaluating the impact of climate change on groundwater resources in a small Mediterranean watershed. Sci Total Environ 499:437–447. https://doi.org/10.1016/j.scitotenv.2014.07.001
Machate O, Schmeller DS, Schulze T, Brack W (2023) Review: mountain lakes as freshwater resources at risk from chemical pollution. Environ Sci Eur 35:3. https://doi.org/10.1186/s12302-022-00710-3
Galli M, Baini M, Panti C et al (2023) Oceanographic and anthropogenic variables driving marine litter distribution in Mediterranean protected areas: extensive field data supported by forecasting modelling. Sci Total Environ 903:166266. https://doi.org/10.1016/j.scitotenv.2023.166266
Feckler A, Wolfram J, Schulz R, Bundschuh M (2023) Reducing pollution to levels not harming biodiversity and ecosystem functions: a perspective on the post-2020 Global Biodiversity Framework. Curr Opin Environ Sci Health 35:100495. https://doi.org/10.1016/j.coesh.2023.100495
Pei T, Shi F, Hou D et al (2023) Enhanced adsorption of phenol from aqueous solution by KOH combined Fe-Zn bimetallic oxide co-pyrolysis biochar: fabrication, performance, and mechanism. Bioresour Technol 388:129746. https://doi.org/10.1016/j.biortech.2023.129746
Mahmoudian MH, Azari A, Jahantigh A et al (2023) Statistical modeling and optimization of dexamethasone adsorption from aqueous solution by Fe3O4@NH2-MIL88B nanorods: isotherm, kinetics, and thermodynamic. Environ Res 236:116773. https://doi.org/10.1016/j.envres.2023.116773
Obayomi KS, Yon Lau S, Danquah MK et al (2023) Selective adsorption of organic dyes from aqueous environment using fermented maize extract-enhanced graphene oxide-durian shell derived activated carbon composite. Chemosphere 339:139742. https://doi.org/10.1016/j.chemosphere.2023.139742
Yu S-H, Wang Y, Wan Y-Y, Guo J-K (2023) Enhance antimony adsorption from aquatic environment by microwave-assisted prepared Fe3O4 nanospherolites. Environ Sci Pollut Res 30:94401–94413. https://doi.org/10.1007/s11356-023-29060-0
Temim D, Guergazi S, Goudjil S, Youcef L (2022) Removal the pollutants of oil mill wastewater by coagulation–flocculation in order to protect the aquatic environment: sludge characterization. Chem Pap 76:6891–6902. https://doi.org/10.1007/s11696-022-02324-8
Pani N, T. S. AS, Menon PM, et al (2022) Electrocoagulation followed by sound agitation for removal of nitrogen and carbon-based pollutants from industrial wastewater. Water Sci Technol 86:2861–2877. https://doi.org/10.2166/wst.2022.364
El Gaayda J, Rachid Y, Titchou FE et al (2023) Optimizing removal of chromium (VI) ions from water by coagulation process using central composite design: effectiveness of grape seed as a green coagulant. Sep Purif Technol 307:122805. https://doi.org/10.1016/j.seppur.2022.122805
Gao W, Mo A, Jiang J et al (2023) Removal of microplastics from water by coagulation of cationic-modified starch: an environmentally friendly solution. Sci Total Environ 904:166787. https://doi.org/10.1016/j.scitotenv.2023.166787
Lashgari M, Naseri-Moghanlou S, Khanahmadlou T, Hempelmann R (2023) Electrostatic boosting of ionic dye pollutant removal from aquatic environment using a single electrode photoreactor. NPJ Clean Water 6:10. https://doi.org/10.1038/s41545-023-00230-4
Kanafin YN, Satayeva A, Abdirova P et al (2023) Membrane bioreactor and advanced oxidation processes for combined treatment of synthetic wastewater containing naproxen, bisphenol A, and sulfamethoxazole. J Water Process Eng 55:104250. https://doi.org/10.1016/j.jwpe.2023.104250
Chianese S, Fenti A, Blotevogel J et al (2023) Trimethoprim removal from wastewater: adsorption and electro-oxidation comparative case study. Case Stud Chem Environ Eng 8:100433. https://doi.org/10.1016/j.cscee.2023.100433
Lei Q, Zhong J, Chen S-F et al (2023) Microbial degradation as a powerful weapon in the removal of sulfonylurea herbicides. Environ Res 235:116570. https://doi.org/10.1016/j.envres.2023.116570
Amaral ET, Bender LBYC, Rizzetti TM (2023) Removal of organic contaminants in water bodies or wastewater by microalgae of the genus Chlorella: a review. Case Stud Chem Environ Eng 8:100476. https://doi.org/10.1016/j.cscee.2023.100476
Subashchandrabose SR, Ramakrishnan B, Megharaj M et al (2013) Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ Int 51:59–72. https://doi.org/10.1016/j.envint.2012.10.007
Kadja GTM, Dwihermiati E, Sagita F et al (2023) Mercapto functionalized–natural zeolites/PVDF mixed matrix membrane for enhanced removal of methylene blue. Inorg Chem Commun 157:111263. https://doi.org/10.1016/j.inoche.2023.111263
Umam K, Sagita F, Pramono E et al (2023) Polyvinylidenefluoride (PVDF)/surface functionalized-mordenite mixed matrix membrane for congo red dyes removal: effect of types of organosilane. JCIS Open 11:100093. https://doi.org/10.1016/j.jciso.2023.100093
Babel S (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243. https://doi.org/10.1016/S0304-3894(02)00263-7
Mastinu K, Maccarinelli et al (2019) Zeolite clinoptilolite: therapeutic virtues of an ancient mineral. Molecules 24:1517. https://doi.org/10.3390/molecules24081517
Pérez-Botella E, Valencia S, Rey F (2022) Zeolites in adsorption processes: state of the art and future prospects. Chem Rev 122:17647–17695. https://doi.org/10.1021/acs.chemrev.2c00140
Grela A, Kuc J, Bajda T (2021) A review on the application of zeolites and mesoporous silica materials in the removal of non-steroidal anti-inflammatory drugs and antibiotics from water. Materials 14:4994. https://doi.org/10.3390/ma14174994
Dyer A (2007) Ion-exchange properties of zeolites and related materials. pp 525–553
Mukaromah AH, Kadja GTM, Mukti RR et al (2016) The Surface-to-volume ratio of the synthesis reactor vessel governing the low temperature crystallization of ZSM-5. J Math Fundam Sci 48:241–251. https://doi.org/10.5614/j.math.fund.sci.2016.48.3.5
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18:1501–1507. https://doi.org/10.1016/0043-1354(84)90124-6
Meiramkulova K, Kydyrbekova A, Devrishov D et al (2023) Comparative analysis of natural and synthetic zeolite filter performance in the purification of groundwater. Water (Basel) 15:588. https://doi.org/10.3390/w15030588
Fajar ATN, Nurdin FA, Mukti RR et al (2020) Synergistic effect of dealumination and ceria impregnation to the catalytic properties of MOR zeolite. Mater Today Chem 17:100313. https://doi.org/10.1016/j.mtchem.2020.100313
Khaleque A, Alam MM, Hoque M et al (2020) Zeolite synthesis from low-cost materials and environmental applications: a review. Environ Adv 2:100019. https://doi.org/10.1016/j.envadv.2020.100019
Wang S, Peng Y (2010) Natural zeolites as effective adsorbents in water and wastewater treatment. Chem Eng J 156:11–24. https://doi.org/10.1016/j.cej.2009.10.029
Aulia D, Culsum NTU, Kadja GTM et al (2024) Current progress in the synthesis of zeolite crystals at low temperatures and their catalytic applications. J Nanopart Res 26:79. https://doi.org/10.1007/s11051-024-05976-7
Le TT, Shilpa K, Lee C et al (2022) Core-shell and egg-shell zeolite catalysts for enhanced hydrocarbon processing. J Catal 405:664–675. https://doi.org/10.1016/j.jcat.2021.11.004
Wang W, He L, Luo Q et al (2023) Synthesis and application of core-shell, hollow, yolk-shell multifunctional structure zeolites. Microporous Mesoporous Mater 362:112766. https://doi.org/10.1016/j.micromeso.2023.112766
Dehmani Y, Ba Mohammed B, Oukhrib R et al (2024) Adsorption of various inorganic and organic pollutants by natural and synthetic zeolites: a critical review. Arab J Chem 17:105474. https://doi.org/10.1016/j.arabjc.2023.105474
Ismail UM, Vohra MS, Onaizi SA (2024) Adsorptive removal of heavy metals from aqueous solutions: progress of adsorbents development and their effectiveness. Environ Res 251:118562. https://doi.org/10.1016/j.envres.2024.118562
Hassani A, Khataee A, Karaca S, Shirzad-Siboni M (2015) Surfactant-modified montmorillonite as a nanosized adsorbent for removal of an insecticide: kinetic and isotherm studies. Environ Technol 36:3125–3135. https://doi.org/10.1080/09593330.2015.1054319
(2020) From weak interactions to covalent bonds: weak, moderate and strong hydrogen bonds. In: Understanding hydrogen bonds. The Royal Society of Chemistry, pp 334–367
Liu H, Kang R, Zhou H et al (2023) Selective adsorption to benzene-cyclohexane gas-phase mixture over ion exchange Y zeolites. Microporous Mesoporous Mater 348:112405. https://doi.org/10.1016/j.micromeso.2022.112405
de Gennaro B (2019) Surface modification of zeolites for environmental applications. In: Modified clay and zeolite nanocomposite materials. Elsevier, pp 57–85
Lu W, Zhang C, Su P et al (2022) Research progress of modified natural zeolites for removal of typical anions in water. Environ Sci 8:2170–2189. https://doi.org/10.1039/D2EW00478J
Wiśniewska M, Urban T, Chibowski S et al (2020) Investigation of adsorption mechanism of phosphate(V) ions on the nanostructured Na-A zeolite surface modified with ionic polyacrylamide with regard to their removal from aqueous solution. Appl Nanosci 10:4475–4485. https://doi.org/10.1007/s13204-020-01397-9
Bajpai SK, Johnson S (2007) Removal of Cr(VI) oxy-anions from aqueous solution by sorption into poly(acrylamide-co-maleic acid) hydrogels. Sep Sci Technol 42:1049–1064. https://doi.org/10.1080/01496390601120623
Velarde L, Nabavi MS, Escalera E et al (2023) Adsorption of heavy metals on natural zeolites: a review. Chemosphere 328:138508. https://doi.org/10.1016/j.chemosphere.2023.138508
Senila M, Neag E, Cadar O et al (2022) Simultaneous removal of heavy metals (Cu, Cd, Cr, Ni, Zn and Pb) from aqueous solutions using thermally treated romanian zeolitic volcanic tuff. Molecules 27:3938. https://doi.org/10.3390/molecules27123938
Mozgawa W, Bajda T (2005) Spectroscopic study of heavy metals sorption on clinoptilolite. Phys Chem Miner 31:706–713. https://doi.org/10.1007/s00269-004-0433-8
Madhusudan P, Lee C, Kim J-O (2023) Synthesis of Al2O3@Fe2O3 core–shell nanorods and its potential for fast phosphate recovery and adsorption of chromium (VI) ions from contaminated wastewater. Sep Purif Technol 326:124691. https://doi.org/10.1016/j.seppur.2023.124691
Miyamoto M, Ono S, Kusukami K et al (2018) High water tolerance of a core–shell-structured zeolite for CO2 adsorptive separation under wet conditions. Chemsuschem 11:1756–1760. https://doi.org/10.1002/cssc.201800063
Rahmah W, Kadja GTM, Mahyuddin MH et al (2022) Small-pore zeolite and zeotype membranes for CO2 capture and sequestration – A review. J Environ Chem Eng 10:108707. https://doi.org/10.1016/j.jece.2022.108707
Wen X, Liang D, Hu Y et al (2023) Performance and mechanism of simultaneous nitrification and denitrification in zeolite spheres internal loop airlift reactor. Bioresour Technol 380:129073. https://doi.org/10.1016/j.biortech.2023.129073
Hou L, Li J, Lu Z (2019) Hollow-structured pollucite microspheres and the formation mechanism. Microporous Mesoporous Mater 282:228–236. https://doi.org/10.1016/j.micromeso.2019.03.037
Yadav VK, Yadav KK, Tirth V et al (2021) Recent advances in methods for recovery of cenospheres from fly ash and their emerging applications in ceramics, composites, polymers and environmental cleanup. Crystals (Basel) 11:1067. https://doi.org/10.3390/cryst11091067
Kumar B, Radhika R, Kumar V, Chakradhar B (2020) Regulatory requirements for fly ash utilisation from thermal power plants in India. Int J Appl Environ Sci 15:151–166
Yadav VK, Fulekar MH (2020) Advances in methods for recovery of ferrous, alumina, and silica nanoparticles from fly ash waste. Ceramics 3:384–420. https://doi.org/10.3390/ceramics3030034
Choudhary N, Yadav VK, Malik P, et al (2020) Recovery of natural nanostructured minerals. pp 450–470
Sun B, Guo Y, Xu L et al (2012) Synthesis of zeolite/mesoporous silica composite microspheres by microemulsion method. Acta Chimi Sin 70:2419. https://doi.org/10.6023/A12080533
Dou B, Li J, Hu Q et al (2010) Hydrophobic micro/mesoporous silica spheres assembled from zeolite precursors in acidic media for aromatics adsorption. Microporous Mesoporous Mater 133:115–123. https://doi.org/10.1016/j.micromeso.2010.04.023
Galogahi FM, Zhu Y, An H, Nguyen N-T (2020) Core-shell microparticles: generation approaches and applications. J Sci Adv Mater Devices 5:417–435. https://doi.org/10.1016/j.jsamd.2020.09.001
Adamiano A, Iafisco M, Tampieri A (2018) Magnetic core-shell nanoparticles. In: Core-shell nanostructures for drug delivery and theranostics. Elsevier pp 259–296
Nomoev AV, Bardakhanov SP, Schreiber M et al (2015) Structure and mechanism of the formation of core–shell nanoparticles obtained through a one-step gas-phase synthesis by electron beam evaporation. Beilstein J Nanotechnol 6:874–880. https://doi.org/10.3762/bjnano.6.89
Strickler AL, Escudero-Escribano M, Jaramillo TF (2017) Core–shell Au@metal-oxide nanoparticle electrocatalysts for enhanced oxygen evolution. Nano Lett 17:6040–6046. https://doi.org/10.1021/acs.nanolett.7b02357
Wang Z, Wan W, Sun J et al (2012) Epitaxial growth of core–shell zeolite X-A composites. CrystEngComm 14:2204. https://doi.org/10.1039/c2ce06432d
Zhang Y, Lin X, Hu S et al (2016) Core–shell zeolite@Alg–Ca particles for removal of strontium from aqueous solutions. RSC Adv 6:73959–73973. https://doi.org/10.1039/C6RA11112B
Tiwari A, Tripathi AK, Khare P (2021) A comprehensive study of synthesis and applications of core/shell nanoparticles. Int J Eng Sci Technol 13:153–157. https://doi.org/10.4314/ijest.v13i1.23S
Deng Y, Deng C, Qi D et al (2009) Synthesis of core/shell colloidal magnetic zeolite microspheres for the immobilization of trypsin. Adv Mater 21:1377–1382. https://doi.org/10.1002/adma.200801766
Wang Z, Wang W, Meng Z, Xue M (2022) Mono-sized anion-exchange magnetic microspheres for protein adsorption. Int J Mol Sci 23:4963. https://doi.org/10.3390/ijms23094963
Piva DH, Piva RH, Ghojavand S et al (2023) Synthesis of core–shell magnetic nanoparticles containing ultrasmall domains of silicalite-1. Adv Mater Interfaces 10:202201961. https://doi.org/10.1002/admi.202201961
Wang C, Yu J, Feng K et al (2022) Synthesis of porous magnetic zeolite-based material and its performance on removal of Cd2+ ion and methylene blue from aqueous solution. Microporous Mesoporous Mater 345:112256. https://doi.org/10.1016/j.micromeso.2022.112256
Abramson S, Safraou W, Malezieux B et al (2011) An eco-friendly route to magnetic silica microspheres and nanospheres. J Colloid Interface Sci 364:324–332. https://doi.org/10.1016/j.jcis.2011.08.056
Shubair T, Eljamal O, Tahara A et al (2019) Preparation of new magnetic zeolite nanocomposites for removal of strontium from polluted waters. J Mol Liq 288:111026. https://doi.org/10.1016/j.molliq.2019.111026
Karimi Pasandideh E, Kakavandi B, Nasseri S et al (2016) Silica-coated magnetite nanoparticles core-shell spheres (Fe3O4@SiO2) for natural organic matter removal. J Environ Health Sci Eng 14:21. https://doi.org/10.1186/s40201-016-0262-y
Shaw R, Madan S, Tiwari S, Tiwari SK (2020) Creating synergy of microporous hydrophilic–organophilic surfaces in zeolite@nano-titania particles for rapid removal of diverse pollutants in water. Adv Mater Interfaces 7:2001386. https://doi.org/10.1002/admi.202001386
Boer DG, Asgar Pour Z, Poli S et al (2023) ZSM-5/silicalite-1 core-shell beads as CO2 adsorbents with increased hydrophobicity. Mater Today Chem 32:101621. https://doi.org/10.1016/j.mtchem.2023.101621
Shamsayei M, Yamini Y, Asiabi H (2022) Synthesis and characterization of layered double hydroxide decorated zeolite as the efficient sorbent for removal of toxic metal ions. Environ Prog Sustain Energy 41:e13727. https://doi.org/10.1002/ep.13727
Lu S, Liu Q, Han R et al (2021) Core-shell structured Y zeolite/hydrophobic organic polymer with improved toluene adsorption capacity under dry and wet conditions. Chem Eng J 409:128194. https://doi.org/10.1016/j.cej.2020.128194
Benkhaya S, M’rabet S, El Harfi A (2020) Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 6:e03271. https://doi.org/10.1016/j.heliyon.2020.e03271
Goh K-H, Lim T-T, Dong Z (2008) Application of layered double hydroxides for removal of oxyanions: a review. Water Res 42:1343–1368. https://doi.org/10.1016/j.watres.2007.10.043
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112:4124–4155. https://doi.org/10.1021/cr200434v
Shamsayei M, Yamini Y, Asiabi H (2018) Evaluation of reusable organic-inorganic nafion/layered double hydroxide nanohybrids for highly efficient uptake of mercury ions from aqueous solution. Appl Clay Sci 162:534–542. https://doi.org/10.1016/j.clay.2018.05.022
Li R, Xue T, Bingre R et al (2018) Microporous zeolite@vertically aligned Mg–Al layered double hydroxide core@shell structures with improved hydrophobicity and toluene adsorption capacity under wet conditions. ACS Appl Mater Interfaces 10:34834–34839. https://doi.org/10.1021/acsami.8b15118
Karim AV, Hassani A, Eghbali P, Nidheesh PV (2022) Nanostructured modified layered double hydroxides (LDHs)-based catalysts: a review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr Opin Solid State Mater Sci 26:100965. https://doi.org/10.1016/j.cossms.2021.100965
John J, Ramesh K, Velayudhaperumal Chellam P (2022) Metal-organic frameworks (MOFs) as a catalyst for advanced oxidation processes—micropollutant removal. In: Advanced Materials for Sustainable Environmental Remediation. Elsevier pp 155–174
Pelalak R, Hassani A, Heidari Z, Zhou M (2023) State-of-the-art recent applications of layered double hydroxides (LDHs) material in Fenton-based oxidation processes for water and wastewater treatment. Chem Eng J 474:145511. https://doi.org/10.1016/j.cej.2023.145511
Al-Naddaf Q, Thakkar H, Rezaei F (2018) Novel zeolite-5A@MOF-74 composite adsorbents with core–shell structure for H2 purification. ACS Appl Mater Interfaces 10:29656–29666. https://doi.org/10.1021/acsami.8b10494
Brazovskaya EYu, Golubeva OYu (2020) Development of magnetic nanocomposites based on beta zeolites and study of their sorption properties. Pet Chem 60:957–963. https://doi.org/10.1134/S0965544120080046
Markandeya Shukla SP, Dhiman N et al (2017) An efficient removal of disperse dye from wastewater using zeolite synthesized from cenospheres. J Hazard Toxic Radioact Waste 21:04017017. https://doi.org/10.1061/(ASCE)HZ.2153-5515.0000369
Singh NJ, Wareppam B, Kumar A et al (2022) Zeolite incorporated iron oxide nanoparticle composites for enhanced congo red dye removal. J Mater Res. https://doi.org/10.1557/s43578-022-00859-w
Imessaoudene A, Cheikh S, Hadadi A et al (2023) Adsorption performance of zeolite for the removal of congo red dye: factorial design experiments, kinetic, and equilibrium studies. Separations 10:57. https://doi.org/10.3390/separations10010057
Coşkun R, Öncel K, Akköz Y (2021) Multifunctional amphoteric resin to remove both anionic and cationic dyes from aqueous solution. J Appl Polym Sci 138:51283. https://doi.org/10.1002/app.51283
Ibrahim MA, Salama A, Zahran F et al (2024) Fabrication of cellulose nanocrystals/carboxymethyl cellulose/zeolite membranes for methylene blue dye removal: understanding factors, adsorption kinetics, and thermodynamic isotherms. Front Chem 12:1330810. https://doi.org/10.3389/fchem.2024.1330810
Raychaudhuri S Sen, Pramanick P, Talukder P, Basak A (2021) Polyamines, metallothioneins, and phytochelatins—natural defense of plants to mitigate heavy metals. pp 227–261
Hawkes SJ (1997) What is a “heavy metal”? J Chem Educ 74:1374. https://doi.org/10.1021/ed074p1374
Mitra S, Chakraborty AJ, Tareq AM et al (2022) Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J King Saud Univ Sci 34:101865. https://doi.org/10.1016/j.jksus.2022.101865
Shishehbore MR, Safaei M (2022) The use of FNMs-based electrochemical sensors in the food and beverage industry. In: Functionalized nanomaterial-based electrochemical sensors. Elsevier, pp 207–259
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691. https://doi.org/10.1016/j.heliyon.2020.e04691
Hong M, Yu L, Wang Y et al (2019) Heavy metal adsorption with zeolites: the role of hierarchical pore architecture. Chem Eng J 359:363–372. https://doi.org/10.1016/j.cej.2018.11.087
Kadja GTM, Azhari NJ, Mardiana S et al (2021) Accelerated, mesoporogen-free synthesis of hierarchical nanorod ZSM-48 assisted by hydroxyl radicals. Ind Eng Chem Res 60:17786–17791. https://doi.org/10.1021/acs.iecr.1c03586
Kithinji Kinoti I, Ogunah J, Muturia M’Thiruaine C, Marangu JM (2022) Adsorption of heavy metals in contaminated water using zeolite derived from agro-wastes and clays: a review. J Chem 2022:1–25. https://doi.org/10.1155/2022/4250299
Kuldeyev E, Seitzhanova M, Tanirbergenova S et al (2023) Modifying natural zeolites to improve heavy metal adsorption. Water (Basel) 15:2215. https://doi.org/10.3390/w15122215
Mubarak MF, Mohamed AMG, Keshawy M et al (2022) Adsorption of heavy metals and hardness ions from groundwater onto modified zeolite: batch and column studies. Alex Eng J 61:4189–4207. https://doi.org/10.1016/j.aej.2021.09.041
Cao J, Wang P, Shen J, Sun Q (2020) Core-shell Fe3O4@zeolite NaA as an adsorbent for Cu2+. Materials 13:5047. https://doi.org/10.3390/ma13215047
Ji Y, Zhang X, Gao J et al (2020) Efficiency and mechanisms of cadmium removal via core-shell zeolite/Zn-layer double hydroxides. Ecotoxicol Environ Saf 188:109887. https://doi.org/10.1016/j.ecoenv.2019.109887
Shaw R, Sharma R, Tiwari S, Tiwari SK (2016) Surface engineered zeolite: an active interface for rapid adsorption and degradation of toxic contaminants in water. ACS Appl Mater Interfaces 8:12520–12527. https://doi.org/10.1021/acsami.6b01754
Funding
This research is supported by Inter University Center for Excellence (PUAPT) program, from Ministry of Education, Culture, Research, and Technology (Kemdikbudristek), the Republic of Indonesia.
Author information
Authors and Affiliations
Contributions
S.F.A, A.K., and W.R. wrote and revised the manuscript; H.R. supervised and revised the manuscript; G.T.M.K. concepted the idea, supervised, obtained the funding, and revised the manuscript.
Corresponding author
Ethics declarations
Declarations
Ethical approval.
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agustin, S.F., Kusdiana, A., Rahmah, W. et al. Zeolite-based core–shell adsorbent for the removal of toxic pollutants from aquatic environment: current challenges and opportunities. J Nanopart Res 26, 94 (2024). https://doi.org/10.1007/s11051-024-05996-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-024-05996-3