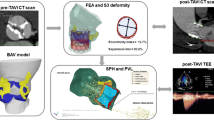
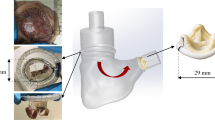
Abstract
Bicuspid aortic valve (BAV), the most common congenital heart malformation, is characterized by the presence of only two valve leaflets with asymmetrical geometry, resulting in elliptical systolic opening. BAV often leads to early onset of calcific aortic stenosis (AS). Following the rapid expansion of transcatheter aortic valve replacement (TAVR), designed specifically for treating conventional tricuspid AS, BAV patients with AS were initially treated “off-label” with TAVR, which recently gained FDA and CE regulatory approval. Despite its increasing use in BAV, pathological BAV anatomy often leads to complications stemming from mismatched anatomical features. To mitigate these complications, a novel eccentric polymeric TAVR valve incorporating asymmetrical leaflets was designed specifically for BAV anatomies. Computational modeling was used to optimize its asymmetric leaflets for lower functional stresses and improved hemodynamic performance. Deployment and flow were simulated in patient-specific BAV models (n = 6) and compared to a current commercial TAVR valve (Evolut R 29 mm), to assess deployment and flow parameters. The novel eccentric BAV-dedicated valve demonstrated significant improvements in peak systolic orifice area, along with lower jet velocity and wall shear stress (WSS). This feasibility study demonstrates the clinical potential of the first known BAV-dedicated TAVR design, which will foster advancement of patient-dedicated valvular devices.








Similar content being viewed by others
Abbreviations
- AP-BAV:
-
Asymmetric polymeric bicuspid aortic valve
- BAV:
-
Bicuspid aortic valve
- CAVD:
-
Calcific aortic valve disease
- CFD:
-
Computational fluid dynamics
- EOA:
-
Effective orifice area
- FEA:
-
Finite element analysis
- TAV:
-
Trileaflet aortic valve
- TAVR:
-
Transcatheter aortic valve replacement
- SAVR:
-
Surgical aortic valve replacement
- WSS:
-
Wall shear stress
References
Abrams J. The aortic valve by Mano Thubrikar Crc Press, Inc., Boca Raton (1990) 221 pages, illustrated, $97.50 ISBN: 0–8493–4771–8. Clin. Cardiol. 1991;14(4):364a–5. https://doi.org/10.1002/clc.4960140417.
Anam, S. B., B. J. Kovarovic, R. P. Ghosh, M. Bianchi, A. Hamdan, R. Haj-Ali, and D. Bluestein. Assessment of paravalvular leak severity and thrombogenic potential in transcatheter bicuspid aortic valve replacements using patient-specific computational modeling. J. Cardiovasc. Transl. Res. 2021. https://doi.org/10.1007/s12265-021-10191-z
Anam, S. B., B. J. Kovarovic, R. P. Ghosh, M. Bianchi, A. Hamdan, R. Haj-Ali, and D. Bluestein. Validating in silico and in vitro patient-specific structural and flow models with transcatheter bicuspid aortic valve replacement procedure. Cardiovasc. Eng. Technol. 2022. https://doi.org/10.1007/s13239-022-00620-8
Appa H, et al. The Technological Basis of a Balloon-Expandable TAVR System: Non-occlusive Deployment, Anchorage in the Absence of Calcification and Polymer Leaflets. Frontiers in Cardiovascular Medicine. 2022;9. doi: https://doi.org/10.3389/fcvm.2022.791949.
Barati, S., N. Fatouraee, M. Nabaei, F. Berti, L. Petrini, F. Migliavacca, and J. F. Rodriguez Matas. A computational optimization study of a self-expandable transcatheter aortic valve. Comput. Biol. Med. 139:104942, 2021. https://doi.org/10.1016/j.compbiomed.2021.104942
Barker CM, von Ballmoos MW, Reardon MJ. Are TAVR Hemodynamics Important in the Lower-Risk Population? Cardiac Interventions Today. 2018, (Mitral Considerations):S3-S6.
Bianchi, M., G. Marom, R. P. Ghosh, H. A. Fernandez, J. R. Taylor Jr., M. J. Slepian, and D. Bluestein. Effect of balloon-expandable transcatheter aortic valve replacement positioning: a patient-specific numerical model. Artif. Organs. 40(12):E292-e304, 2016. https://doi.org/10.1111/aor.12806
Bianchi, M., G. Marom, R. P. Ghosh, O. M. Rotman, P. Parikh, L. Gruberg, and D. Bluestein. Patient-specific simulation of transcatheter aortic valve replacement: impact of deployment options on paravalvular leakage. Biomech. Model. Mechanobiol. 18(2):435–451, 2019.
Bollache, E., et al. Aortic valve-mediated wall shear stress is heterogeneous and predicts regional aortic elastic fiber thinning in bicuspid aortic valve-associated aortopathy. J. Thorac. Cardiovasc. Surg. 156(6):2112–2120, 2018. https://doi.org/10.1016/j.jtcvs.2018.05.095
Caballero, A., F. Sulejmani, C. Martin, T. Pham, and W. Sun. Evaluation of transcatheter heart valve biomaterials: Biomechanical characterization of bovine and porcine pericardium. J. Mech. Behav. Biomed. Mater. 75:486–494, 2017. https://doi.org/10.1016/j.jmbbm.2017.08.013
Carabello, B. A. How does the heart respond to aortic stenosis. Circulation. 6(6):858–60, 2013. https://doi.org/10.1161/CIRCIMAGING.113.001242
Carbonaro, D., D. Gallo, U. Morbiducci, A. Audenino, and C. Chiastra. In silico biomechanical design of the metal frame of transcatheter aortic valves: multi-objective shape and cross-sectional size optimization. Struct. Multidisc. Optim. 2021. https://doi.org/10.1007/s00158-021-02944-w
Claiborne, T. E., et al. Toward optimization of a novel trileaflet polymeric prosthetic heart valve via device thrombogenicity emulation. ASAIO J. 59(3):275–83, 2013. https://doi.org/10.1097/MAT.0b013e31828e4d80
Claiborne, T. E., J. Sheriff, M. Kuetting, U. Steinseifer, M. J. Slepian, and D. Bluestein. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. J. Biomech. Eng. 135(2):021021, 2013. https://doi.org/10.1115/1.4023235
Claiborne, T. E., M. J. Slepian, S. Hossainy, and D. Bluestein. Polymeric trileaflet prosthetic heart valves: evolution and path to clinical reality. Expert Rev Med Devices. 9(6):577–594, 2012. https://doi.org/10.1586/erd.12.51
Durko, A. P., R. L. Osnabrugge, and A. P. Kappetein. Long-term outlook for transcatheter aortic valve replacement. Trends Cardiovasc. Med. 28(3):174–183, 2018. https://doi.org/10.1016/j.tcm.2017.08.004
Egron, S., B. Fujita, L. Gullón, D. Pott, T. Schmitz-Rode, S. Ensminger, and U. Steinseifer. Radial force: an underestimated parameter in oversizing transcatheter aortic valve replacement prostheses: in vitro analysis with five commercialized valves. ASAIO J. 64(4):536–43, 2018. https://doi.org/10.1097/mat.0000000000000659
Emendi, M., et al. Patient-specific bicuspid aortic valve biomechanics: a magnetic resonance imaging integrated fluid-structure interaction approach. Ann. Biomed. Eng. 49(2):627–641, 2021. https://doi.org/10.1007/s10439-020-02571-4
Ghosh, R. P., G. Marom, M. Bianchi, K. D’Souza, W. Zietak, and D. Bluestein. Numerical evaluation of transcatheter aortic valve performance during heart beating and its post-deployment fluid-structure interaction analysis. Biomech. Model. Mechanobiol. 2020. https://doi.org/10.1007/s10237-020-01304-9
Ghosh, R., G. Marom, O. Rotman, M. J. Slepian, S. Prabhakar, M. Horner, and D. Bluestein. Comparative fluid-structure interaction analysis of polymeric transcatheter and surgical aortic valves’ hemodynamics and structural mechanics. J. Biomech. Eng. 2018. https://doi.org/10.1115/1.4040600
Iannopollo, G., et al. Supra-annular sizing of transcatheter aortic valve prostheses in raphe-type bicuspid aortic valve disease: the LIRA method. Int. J. Cardiol. 317:144–151, 2020. https://doi.org/10.1016/j.ijcard.2020.05.076
Kovarovic, B., R. Helbock, K. Baylous, O. M. Rotman, M. J. Slepian, and D. Bluestein. Visions of TAVR future: development and optimization of a second generation novel polymeric TAVR. J. Biomech. Eng. 2022. https://doi.org/10.1115/1.4054149
Lavon, K., G. Marom, M. Bianchi, R. Halevi, A. Hamdan, A. Morany, E. Raanani, D. Bluestein, and R. Haj-Ali. Biomechanical modeling of transcatheter aortic valve replacement in a stenotic bicuspid aortic valve: deployments and paravalvular leakage. Med. Biol. Eng. Comput. 57(10):2129–2143, 2019. https://doi.org/10.1007/s11517-019-02012-y
Lavon, K., A. Morany, R. Halevi, A. Hamdan, E. Raanani, D. Bluestein, and R. Haj-Ali. Progressive calcification in bicuspid valves: a coupled hemodynamics and multiscale structural computations. Ann. Biomed. Eng. 49(12):3310–22, 2021. https://doi.org/10.1007/s10439-021-02877-x
Marom, G., H.-S. Kim, M. Rosenfeld, E. Raanani, and R. Haj-Ali. Fully coupled fluid–structure interaction model of congenital bicuspid aortic valves: effect of asymmetry on hemodynamics. Med. Biol. Eng. Comput. 51(8):839–848, 2013. https://doi.org/10.1007/s11517-013-1055-4
Martin, C., T. Pham, and W. Sun. Significant differences in the material properties between aged human and porcine aortic tissues. Eur. J. Cardiothorac. Surg. 40(1):28–34, 2011. https://doi.org/10.1016/j.ejcts.2010.08.056
Martin, C., and W. Sun. Biomechanical characterization of aortic valve tissue in humans and common animal models. J. Biomed. Mater. Res. A. 100(6):1591–1599, 2012. https://doi.org/10.1002/jbm.a.34099
Martin, C., and W. Sun. Transcatheter valve underexpansion limits leaflet durability: implications for valve-in-valve procedures. Ann. Biomed. Eng. 45(2):394–404, 2017. https://doi.org/10.1007/s10439-016-1738-8
Meierhofer, C., et al. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur. Heart J. Cardiovasc. Imaging. 14(8):797–804, 2012. https://doi.org/10.1093/ehjci/jes273
Rocatello, G., G. De Santis, S. De Bock, M. De Beule, P. Segers, and P. Mortier. Optimization of a transcatheter heart valve frame using patient-specific computer simulation. Cardiovasc. Eng. Technol. 10(3):456–468, 2019. https://doi.org/10.1007/s13239-019-00420-7
Rodrigues, I., et al. Bicuspid aortic valve outcomes. Cardiol. Young. 27(3):518–529, 2017. https://doi.org/10.1017/s1047951116002560
Rotman, O. M., M. Bianchi, R. P. Ghosh, B. Kovarovic, and D. Bluestein. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices. 15(11):771–791, 2018. https://doi.org/10.1080/17434440.2018.1536427
Rotman, O. M., B. Kovarovic, M. Bianchi, M. J. Slepian, and D. Bluestein. In vitro durability and stability testing of a novel polymeric transcatheter aortic valve. ASAIO J. 66(2):190–8, 2020. https://doi.org/10.1097/mat.0000000000000980
Rotman, O. M., B. Kovarovic, W. C. Chiu, M. Bianchi, G. Marom, M. J. Slepian, and D. Bluestein. Novel polymeric valve for transcatheter aortic valve replacement applications: in vitro hemodynamic study. Ann. Biomed. Eng. 47(1):113–25, 2019. https://doi.org/10.1007/s10439-018-02119-7
Shibayama, K., K. Harada, J. Berdejo, J. Tanaka, H. Mihara, Y. Itabashi, and T. Shiota. Comparison of aortic root geometry with bicuspid versus tricuspid aortic valve: real-time three-dimensional transesophageal echocardiographic study. J. Am. Soc. Echocardiogr. 27(11):1143–1152, 2014. https://doi.org/10.1016/j.echo.2014.07.008
Siu, S. C., and C. K. Silversides. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 55(25):2789, 2010. https://doi.org/10.1016/j.jacc.2009.12.068
Tchetche, D., et al. Bicuspid aortic valve anatomy and relationship with devices: The BAVARD Multicenter Registry. Circulation. 12(1):e007107, 2019. https://doi.org/10.1161/CIRCINTERVENTIONS.118.007107
Vincent, F., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 143(10):1043–1061, 2021. https://doi.org/10.1161/CIRCULATIONAHA.120.048048
Yan, W., J. Li, W. Wang, L. Wei, and S. Wang. A fluid-structure interaction study of different bicuspid aortic valve phenotypes throughout the cardiac cycle. Front. Physiol. 2021. https://doi.org/10.3389/fphys.2021.716015
Yoganathan, A. P. Fluid mechanics of aortic stenosis. Eur. Heart J. 9 Suppl E:13–7, 1988. https://doi.org/10.1093/eurheartj/9.suppl_e.13
Yoon, S.-H., et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 76(9):1018–1030, 2020. https://doi.org/10.1016/j.jacc.2020.07.005
Acknowledgments
The authors would like to thank the continued research collaboration with the Simulia Living Heart Project (Dassault Systemes), ANSYS software, and SeaWulf Cluster at Stony Brook University for providing computational resources. This project was supported by NIH-NIBIB-BRPU01EB026414 (DB), and STTR-R42HL134418-03A1.
Conflict of interest
Authors DB and MJS have an equity interest in PolyNova Cardiovascular Inc. Author BK is a consultant of Polynova Cardiovascular Inc. All the other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (AVI 3307 kb).
Supplementary file2 (AVI 19572 kb).
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Helbock, R.T., Anam, S.B., Kovarovic, B.J. et al. Designing a Novel Asymmetric Transcatheter Aortic Valve for Stenotic Bicuspid Aortic Valves Using Patient-Specific Computational Modeling. Ann Biomed Eng 51, 58–70 (2023). https://doi.org/10.1007/s10439-022-03039-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-022-03039-3