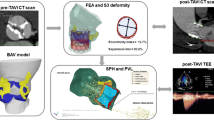
Abstract
Introduction
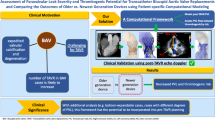
Bicuspid aortic valve (BAV) is the most common congenital cardiac malformation, which had been treated off-label by transcatheter aortic valve replacement (TAVR) procedure for several years, until its recent approval by the Food and Drug Administration (FDA) and Conformité Européenne (CE) to treat BAVs. Post-TAVR complications tend to get exacerbated in BAV patients due to their inherent aortic root pathologies. Globally, due to the paucity of randomized clinical trials, clinicians still favor surgical AVR as the primary treatment option for BAV patients. While this warrants longer term studies of TAVR outcomes in BAV patient cohorts, in vitro experiments and in silico computational modeling can be used to guide the surgical community in assessing the feasibility of TAVR in BAV patients. Our goal is to combine these techniques in order to create a modeling framework for optimizing pre-procedural planning and minimize post-procedural complications.
Materials and Methods
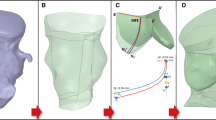
Patient-specific in silico models and 3D printed replicas of 3 BAV patients with different degrees of post-TAVR paravalvular leakage (PVL) were created. Patient-specific TAVR device deployment was modeled in silico and in vitro—following the clinical procedures performed in these patients. Computational fluid dynamics simulations and in vitro flow studies were performed in order to obtain the degrees of PVL in these models.
Results
PVL degree and locations were consistent with the clinical data. Cross-validation comparing the stent deformation and the flow parameters between the in silico and the in vitro models demonstrated good agreement.
Conclusion
The current framework illustrates the potential of using simulations and 3D printed models for pre-TAVR planning and assessing post-TAVR complications in BAV patients.









Similar content being viewed by others
Abbreviations
- BAV:
-
Bicuspid aortic valve
- CFD:
-
Computational fluid dynamics
- FDA:
-
Food and Drug Administration
- FE:
-
Finite element
- LCL:
-
Left coronary leaflet
- LOA:
-
Limit of agreement
- LVOT:
-
Left ventricular outflow tract
- NCL:
-
Non-coronary leaflet
- PVL:
-
Paravalvular leak
- RCL:
-
Right coronary leaflet
- SAVR:
-
Surgical aortic valve replacement
- TAVR:
-
Transcatheter aortic valve replacement
References
Agilus 30. Stratasys Direct Manufacturing. https://www.stratasys.com/materials/search/agilus30.
Anam, S. B., et al. Assessment of paravalvular leak severity and thrombogenic potential in transcatheter bicuspid aortic valve replacements using patient-specific computational modeling. J. Cardiovasc. Transl. Res. 2021. https://doi.org/10.1007/s12265-021-10191-z.
Bailey, J., N. Curzen, and N. W. Bressloff. Assessing the impact of including leaflets in the simulation of TAVI deployment into a patient-specific aortic root. Comput. Methods Biomech. Biomed. Eng. 19:733–744, 2016. https://doi.org/10.1080/10255842.2015.1058928.
Bana, A. TAVR—present, future, and challenges in developing countries. Indian J. Thorac. Cardiovasc. Surg. 35:473–484, 2019. https://doi.org/10.1007/s12055-018-00786-8.
Ben-Dor, I., and A. Stewart. A cautionary tale of 2 leaflets. J. Am. Coll. Cardiol. 69:2590–2591, 2017. https://doi.org/10.1016/j.jacc.2017.03.573.
Bianchi, M., et al. Effect of balloon-expandable transcatheter aortic valve replacement positioning: a patient-specific numerical model. Artif. Organs. 40:E292–E304, 2016. https://doi.org/10.1111/aor.12806.
Bianchi, M., et al. Patient-specific simulation of transcatheter aortic valve replacement: impact of deployment options on paravalvular leakage. Biomech. Model. Mechanobiol. 18:435–451, 2019. https://doi.org/10.1007/s10237-018-1094-8.
Bosi, G. M., et al. A validated computational framework to predict outcomes in TAVI. Sci. Rep. 10:9906, 2020. https://doi.org/10.1038/s41598-020-66899-6.
Braverman, A. C., et al. The bicuspid aortic valve. Curr. Probl. Cardiol. 30:470–522, 2005. https://doi.org/10.1016/j.cpcardiol.2005.06.002.
Chakravarty, T., et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 389:2383–2392, 2017. https://doi.org/10.1016/S0140-6736(17)30757-2.
Conte, S. M., et al. Plugging paravalvular leak in transcatheter aortic valves. JACC Case Rep. 1:696–702, 2019. https://doi.org/10.1016/j.jaccas.2019.10.013.
Davlouros, P. A., V. C. Mplani, I. Koniari, G. Tsigkas, and G. Hahalis. Transcatheter aortic valve replacement and stroke: a comprehensive review. J. Geriatr. Cardiol. 15:95–104, 2018. https://doi.org/10.11909/j.issn.1671-5411.2018.01.008.
Dowling, C., S. Firoozi, and S. J. Brecker. First-in-human experience with patient-specific computer simulation of TAVR in bicuspid aortic valve morphology. JACC Cardiovasc. Interv. 13:184–192, 2020. https://doi.org/10.1016/j.jcin.2019.07.032.
Dutta, P., and J. Lincoln. Calcific aortic valve disease: a developmental biology perspective. Curr. Cardiol. Rep. 20:21, 2018. https://doi.org/10.1007/s11886-018-0968-9.
Emendi, M., et al. Patient-specific bicuspid aortic valve biomechanics: a magnetic resonance imaging integrated fluid–structure interaction approach. Ann. Biomed. Eng. 49:627–641, 2021. https://doi.org/10.1007/s10439-020-02571-4.
Ghosh, R. P., et al. Numerical evaluation of transcatheter aortic valve performance during heart beating and its post-deployment fluid–structure interaction analysis. Biomech. Model. Mechanobiol. 19:1725–1740, 2020. https://doi.org/10.1007/s10237-020-01304-9.
Halim, S. A., et al. Outcomes of transcatheter aortic valve replacement in patients with bicuspid aortic valve disease. Circulation. 141:1071–1079, 2020. https://doi.org/10.1161/CIRCULATIONAHA.119.040333.
Hussein, R., M. Wolbinski, V. Bapat, T. M. Nazif. TAVR for Bicuspid Aortic Valve Disease, 2020. https://citoday.com/articles/2020-mar-apr/tavr-for-bicuspid-aortic-valve-disease. Accessed 15 Aug 2021.
ISO. Cardiovascular Implants—Cardiac Valve Prostheses—Part 3: Heart Valve Substitutes Implanted by Transcatheter Technique. ISO5840-3:2013. Geneva: ISO, 2013.
de Jaegere, P., et al. Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 9:508–512, 2016. https://doi.org/10.1016/j.jcin.2016.01.003.
Keshavarz-Motamed, Z., et al. Mixed valvular disease following transcatheter aortic valve replacement: quantification and systematic differentiation using clinical measurements and image-based patient-specific in silico modeling. J. Am. Heart Assoc.9:e015063, 2020. https://doi.org/10.1161/JAHA.119.015063.
Kong William, K. F., V. Delgado, and J. Bax Jeroen. Bicuspid aortic valve. Circ. Cardiovasc. Imaging. 10:e005987, 2017. https://doi.org/10.1161/CIRCIMAGING.117.005987.
Kovarovic, B. J., O. M. Rotman, P. Parikh, M. J. Slepian, and D. Bluestein. Patient-specific in vitro testing for evaluating TAVR clinical performance—a complementary approach to current ISO standard testing. Artif. Organs. 45:E41–E52, 2021. https://doi.org/10.1111/aor.13841.
Lau, I., and Z. Sun. Three-dimensional printing in congenital heart disease: a systematic review. J. Med. Radiat. Sci. 65:226–236, 2018. https://doi.org/10.1002/jmrs.268.
Lavon, K., et al. Biomechanical modeling of transcatheter aortic valve replacement in a stenotic bicuspid aortic valve: deployments and paravalvular leakage. Med. Biol. Eng. Comput. 57:2129–2143, 2019. https://doi.org/10.1007/s11517-019-02012-y.
Lee, S., A. Squelch, and Z. Sun. Quantitative assessment of 3D printed model accuracy in delineating congenital heart disease. Biomolecules. 2021. https://doi.org/10.3390/biom11020270.
Lee Alex, P.-W., M. Leong Chun Wing, K.-W. Kwok, and Y. Fan. Using 3D printed models for planning transcatheter aortic valve implantation in patients with bicuspid aortic valve. J. Am. Coll. Cardiol. 71:A1130, 2018. https://doi.org/10.1016/S0735-1097(18)31671-1.
Losenno, K. L., R. L. Goodman, and M. W. A. Chu. Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol. Res. Pract.2012:145202, 2012. https://doi.org/10.1155/2012/145202.
Makkar, R., T. Chakravarty, and H. Jilaihawi. Transcatheter aortic valve replacement for bicuspid aortic stenosis: are we ready for the challenge? J. Am. Coll. Cardiol. 68:1206–1208, 2016. https://doi.org/10.1016/j.jacc.2016.06.042.
Malaisrie, S. C., and M. P. McCarthy. TAVR for Bicuspid Aortic Valves: Is Surgery Still the Gold Standard? American College of Cardiology, 2019.
Mangieri, A., et al. Balloon versus self-expandable valve for the treatment of bicuspid aortic valve stenosis. Circ. Cardiovasc. Interv.13:e008714, 2020. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008714.
Mao, W., Q. Wang, S. Kodali, and W. Sun. Numerical parametric study of paravalvular leak following a transcatheter aortic valve deployment into a patient-specific aortic root. J. Biomech. Eng. 140:1010071–10100711, 2018. https://doi.org/10.1115/1.4040457.
Martin, C., T. Pham, and W. Sun. Significant differences in the material properties between aged human and porcine aortic tissues. Eur. J. Cardiothorac. Surg. 40:28–34, 2011. https://doi.org/10.1016/j.ejcts.2010.08.056.
Martin, C., and W. Sun. Biomechanical characterization of aortic valve tissue in humans and common animal models. J. Biomed. Mater. Res. A. 100A:1591–1599, 2012. https://doi.org/10.1002/jbm.a.34099.
Martin, L. C. W. Prediction of Paravalvular Leak after Transcatheter Aortic Valve Implantation using Patient-specific 3D-printed Models - a case with Bicuspid Aortic Valve Stenosis. The Chinese University of Hong Kong, 2017. https://www.ksecho.org:4458/workshop/2017fall_w/file/poster/C-1.pdf. Accessed 15 Aug 2021.
Mauri, V., et al. Impact of device landing zone calcification patterns on paravalvular regurgitation after transcatheter aortic valve replacement with different next-generation devices. Open Heart.7:e001164, 2020. https://doi.org/10.1136/openhrt-2019-001164.
Milhorini Pio, S., J. Bax, and V. Delgado. How valvular calcification can affect the outcomes of transcatheter aortic valve implantation. Expert Rev. Med. Devices. 17:773–784, 2020. https://doi.org/10.1080/17434440.2020.1789456.
Morganti, S., et al. Prediction of patient-specific post-operative outcomes of TAVI procedure: the impact of the positioning strategy on valve performance. J. Biomech. 49:2513–2519, 2016. https://doi.org/10.1016/j.jbiomech.2015.10.048.
O’Riordon, M. Positive Early Data for TAVR in Low-Risk Patients with Bicuspid Valves, 2020. https://www.tctmd.com/news/positive-early-data-tavr-low-risk-patients-bicuspid-valves. Accessed 15 Aug 2021.
Pasta, S., et al. Simulation study of transcatheter heart valve implantation in patients with stenotic bicuspid aortic valve. Med. Biol. Eng. Comput. 58:815–829, 2020. https://doi.org/10.1007/s11517-020-02138-4.
Pibarot, P., T. Hahn Rebecca, J. Weissman Neil, and J. Monaghan Mark. Assessment of paravalvular regurgitation following TAVR. JACC Cardiovasc. Imaging. 8:340–360, 2015. https://doi.org/10.1016/j.jcmg.2015.01.008.
Polyjet Flex and PolyJet Over-mold. Stratasys Direct Manufacturing. https://www.stratasysdirect.com/zh-cn/technologies/polyjet. Accessed 15 Aug 2021.
Qin, T., et al. The role of stress concentration in calcified bicuspid aortic valve. J. R. Soc. Interface. 17:20190893, 2020. https://doi.org/10.1098/rsif.2019.0893.
Ram, D., et al. Concepts of bicuspid aortic valve repair: a review. Ann. Thorac. Surg. 109:999–1006, 2020. https://doi.org/10.1016/j.athoracsur.2019.09.019.
Rotman, O. M., M. Bianchi, R. P. Ghosh, B. Kovarovic, and D. Bluestein. Principles of TAVR valve design, modelling, and testing. Expert Rev. Med. Devices. 15:771–791, 2018. https://doi.org/10.1080/17434440.2018.1536427.
Saad, M., H. Seoudy, and D. Frank. Challenging anatomies for TAVR—bicuspid and beyond. Front. Cardiovasc. Med. 2021. https://doi.org/10.3389/fcvm.2021.654554.
Sievers, H.-H., and C. Schmidtke. A classification system for the bicuspid aortic valve from 304 surgical specimens. J. Thorac. Cardiovasc. Surg. 133:1226–1233, 2007. https://doi.org/10.1016/j.jtcvs.2007.01.039.
Tchetche, D., et al. Bicuspid aortic valve anatomy and relationship with devices: the BAVARD Multicenter Registry. Circ. Cardiovasc. Interv.12:e007107, 2019. https://doi.org/10.1161/circinterventions.118.007107.
Vincent, F., et al. Transcatheter aortic valve replacement in bicuspid aortic valve stenosis. Circulation. 143:1043–1061, 2021. https://doi.org/10.1161/circulationaha.120.048048.
Wang, Q., S. Kodali, C. Primiano, and W. Sun. Simulations of transcatheter aortic valve implantation: implications for aortic root rupture. Biomech. Model. Mechanobiol. 14:29–38, 2015. https://doi.org/10.1007/s10237-014-0583-7.
Ward, C. Clinical significance of the bicuspid aortic valve. Heart. 83:81, 2000. https://doi.org/10.1136/heart.83.1.81.
Yıldırım, A. I., and A. T. Karaağaç. In: Structural Insufficiency Anomalies in Cardiac Valves, edited by K. Kirali. London: InTech, 2018.
Yoon, S. H., et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 76:1018–1030, 2020. https://doi.org/10.1016/j.jacc.2020.07.005.
Yoon, S.-H., and R. R. Makkar. TAVR for Severe Bicuspid Aortic Valve Stenosis. American College of Cardiology, 2018.
Acknowledgments
We would like to thank the SeaWulf Supercomputer at the Institute for Advanced Computational Science at Stony Brook University for providing computational resources. ANSYS and the Dassault Systèmes Simulia Living Heart Project are in academic partnerships with Professor Danny Bluestein.
Funding
This project was supported by NIH-NIBIB U01EB026414 (DB).
Conflict of interest
Author DB has an equity interest in PolyNova Cardiovascular, Inc. Author BK is a consultant of Polynova Cardiovascular, Inc. All the other authors have no conflict of interest.
Ethical Approval
All procedures involving human participants were in accordance with the ethical standards of the Responsible Committee on Human Experimentation (Institutional and National Research) and with the Helsinki Declaration of 1975, as revised in 2000. IRB approval: 2013-2357-R5, 2/10/2021.
Informed Consent
A waiver of consent was approved by the Stony Brook Committee on Research in Human Subjects (CORIHS-2013-2357-F) and Rabin Medical Center Helsinki Committee (0636-16-RMC) for retrospective collection of de-identified patient images. No animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Amy L. Throckmorton oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anam, S.B., Kovarovic, B.J., Ghosh, R.P. et al. Validating In Silico and In Vitro Patient-Specific Structural and Flow Models with Transcatheter Bicuspid Aortic Valve Replacement Procedure. Cardiovasc Eng Tech 13, 840–856 (2022). https://doi.org/10.1007/s13239-022-00620-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-022-00620-8