Abstract
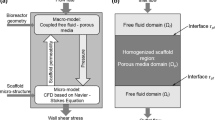
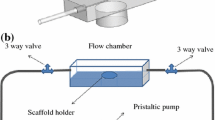
Perfusion bioreactors are known to exert shear stresses on cultured cells, leading to cell differentiation and enhanced extracellular matrix deposition on scaffolds. The influence of the scaffold’s porous microstructure is investigated for a polycaprolactone (PCL) scaffold with a regular microarchitecture and a silk fibroin (SF) scaffold with an irregular network of interconnected pores. Their complex 3D geometries are imaged by micro-computed tomography and used in direct pore-level simulations of the entire scaffold–bioreactor system to numerically solve the governing mass and momentum conservation equations for fluid flow through porous media. The velocity field and wall shear stress distribution are determined for both scaffolds. The PCL scaffold exhibited an asymmetric distribution with peak and plateau, while the SF scaffold exhibited a homogenous distribution and conditioned the flow more efficiently than the PCL scaffold. The methodology guides the design and optimization of the scaffold geometry.






Similar content being viewed by others
Abbreviations
- CFD:
-
Computational fluid dynamics
- micro-CT:
-
Micro-computed tomography
- DPLS:
-
Direct pore-level simulations
- ECM:
-
Extracellular matrix
- PCL:
-
Polycaprolactone
- REV:
-
Representative elementary volume
- ROI:
-
Region of interest
- SF:
-
Silk fibroin
- u :
-
Velocity (m/s)
- y :
-
Height above the phase boundary (m)
- μ :
-
Dynamic viscosity (kg/m/s)
- ρ :
-
Density (kg/m3)
- τ w :
-
Wall shear stress (Pa)
References
Ansys. Ansys-CFX, 2009.
Bancroft, G. N., V. I. Sikavitsas, J. van den Dolder, T. L. Sheffield, C. G. Ambrose, J. A. Jansen, and A. G. Mikos. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl. Acad. Sci. 99(20):12600–12605, 2002.
Biotek, D. 3D InsertTM PCL scaffold.
Boschetti, F., M. T. Raimondi, F. Migliavacca, and G. Dubini. Prediction of the micro-fluid dynamic environment imposed to three-dimensional engineered cell systems in bioreactors. J. Biomech. 39(3):418–425, 2006.
Botchwey, E., S. Pollack, E. Levine, and C. Laurencin. Bone tissue engineering in a rotating bioreactor using a microcarrier matrix system. J. Biomed. Mater. Res. 55(2):242–253, 2001.
Byrne, D. P., D. Lacroix, J. A. Planell, D. J. Kelly, and P. J. Prendergast. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: application of mechanobiological models in tissue engineering. Biomaterials 28(36):5544–5554, 2007.
Cioffi, M., F. Boschetti, M. T. Raimondi, and G. Dubini. Modeling evaluation of the fluid-dynamic microenvironment in tissue-engineered constructs: a micro-CT based model. Biotechnol. Bioeng. 93(3):500–510, 2006.
David, V., A. Guignandon, A. Martin, L. Malaval, M.-H. Lafage-Proust, A. Rattner, V. Mann, B. Noble, D. B. Jones, and L. Vico. Ex vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Eng. Part A 14(1):117–126, 2008.
Fredrich, J., A. DiGiovanni, and D. Noble. Predicting macroscopic transport properties using microscopic image data. J. Geophys. Res. Solid Earth (1978–2012). 111(B3), 2006.
Friess, H., S. Haussener, A. Steinfeld, and J. Petrasch. Tetrahedral mesh generation based on space indicator functions. Int. J. Numer. Methods Eng. 93:1040–1056, 2012.
Goldstein, A. S., T. M. Juarez, C. D. Helmke, M. C. Gustin, and A. G. Mikos. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials 22(11):1279–1288, 2001.
Gomes, M. E., V. I. Sikavitsas, E. Behravesh, R. L. Reis, and A. G. Mikos. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J. Biomed. Mater. Res. Part A 67(1):87–95, 2003.
Haussener, S., P. Coray, W. Lipinski, P. Wyss, and A. Steinfeld. Tomography-based heat and mass transfer characterization of reticulate porous ceramics for high-temperature processing. J. Heat Transfer 132:023305, 2010.
Haussener, S., and A. Steinfeld. Effective heat and mass transport properties of anisotropic porous ceria for solar thermochemical fuel generation. Materials 5(1):192–209, 2012.
Ho, S. T., and D. W. Hutmacher. A comparison of micro CT with other techniques used in the characterization of scaffolds. Biomaterials 27(8):1362–1376, 2006.
Hofmann, S., H. Hagenmüller, A. M. Koch, R. Müller, G. Vunjak-Novakovic, D. L. Kaplan, H. P. Merkle, and L. Meinel. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 28(6):1152–1162, 2007.
Hofmann, S., K. S. Stok, T. Kohler, A. J. Meinel, and R. Müller. Effect of sterilization on structural and material properties of 3-D silk fibroin scaffolds. Acta Biomater. 10(1):308–317, 2014.
Jungreuthmayer, C., S. W. Donahue, M. J. Jaasma, A. A. Al-Munajjed, J. Zanghellini, D. J. Kelly, and F. J. O’Brien. A comparative study of shear stresses in collagen-glycosaminoglycan and calcium phosphate scaffolds in bone tissue-engineering bioreactors. Tissue Eng. Part A 15(5):1141–1149, 2008.
Kelly, D., and P. J. Prendergast. Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J. Biomech. 38(7):1413–1422, 2005.
Lappa, M. Organic tissues in rotating bioreactors: fluid-mechanical aspects, dynamic growth models, and morphological evolution. Biotechnol. Bioeng. 84(5):518–532, 2003.
Lesman, A., Y. Blinder, and S. Levenberg. Modeling of flow-induced shear stress applied on 3D cellular scaffolds: implications for vascular tissue engineering. Biotechnol. Bioeng. 105(3):645–654, 2010.
Maes, F., T. Claessens, M. Moesen, H. Van Oosterwyck, P. Van Ransbeeck, and P. Verdonck. Computational models for wall shear stress estimation in scaffolds: a comparative study of two complete geometries. J. Biomech. 45(9):1586–1592, 2012.
Maes, F., P. Van Ransbeeck, H. Van Oosterwyck, and P. Verdonck. Modeling fluid flow through irregular scaffolds for perfusion bioreactors. Biotechnol. Bioeng. 103(3):621–630, 2009.
McGarry, J. G., J. Klein-Nulend, M. G. Mullender, and P. J. Prendergast. A comparison of strain and fluid shear stress in stimulating bone cell responses—a computational and experimental study. FASEB J. 19(3):482–484, 2005.
Milan, J.-L., J. A. Planell, and D. Lacroix. Computational modelling of the mechanical environment of osteogenesis within a polylactic acid–calcium phosphate glass scaffold. Biomaterials 30(25):4219–4226, 2009.
Milan, J.-L., J. A. Planell, and D. Lacroix. Simulation of bone tissue formation within a porous scaffold under dynamic compression. Biomech. Model. Mechanobiol. 9(5):583–596, 2010.
Nazarov, R., H.-J. Jin, and D. L. Kaplan. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules 5(3):718–726, 2004.
Nerem, R. Tissue engineering in the USA. Med. Biol. Eng. Comput. 30(4):CE8–CE12, 1992.
Nichol, J. W., and A. Khademhosseini. Modular tissue engineering: engineering biological tissues from the bottom up. Soft Matter 5(7):1312–1319, 2009.
Olivares, A. L., È. Marsal, J. A. Planell, and D. Lacroix. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 30(30):6142–6149, 2009.
Petrasch, J., P. Wyss, and A. Steinfeld. Tomography-based Monte Carlo determination of radiative properties of reticulate porous ceramics. J. Quant. Spectrosc. Radiat. Transfer 105(2):180–197, 2007.
Place, E. S., N. D. Evans, and M. M. Stevens. Complexity in biomaterials for tissue engineering. Nat. Mater. 8(6):457–470, 2009.
Porter, B., R. Zauel, H. Stockman, R. Guldberg, and D. Fyhrie. 3-D computational modeling of media flow through scaffolds in a perfusion bioreactor. J. Biomech. 38(3):543–549, 2005.
Sandino, C., and D. Lacroix. A dynamical study of the mechanical stimuli and tissue differentiation within a CaP scaffold based on micro-CT finite element models. Biomech. Model. Mechanobiol. 10(4):565–576, 2011.
Sandino, C., J. Planell, and D. Lacroix. A finite element study of mechanical stimuli in scaffolds for bone tissue engineering. J. Biomech. 41(5):1005–1014, 2008.
Sikavitsas, V. I., G. N. Bancroft, H. L. Holtorf, J. A. Jansen, and A. G. Mikos. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc. Natl. Acad. Sci. 100(25):14683–14688, 2003.
Sikavitsas, V. I., G. N. Bancroft, J. J. Lemoine, M. A. Liebschner, M. Dauner, and A. G. Mikos. Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann. Biomed. Eng. 33(1):63–70, 2005.
Tsukada, M., Y. Gotoh, M. Nagura, N. Minoura, N. Kasai, and G. Freddi. Structural-changes of silk fibroin membranes induced by immersion in methanol aqueous-solutions. J. Polym. Sci. B 32(5):961–968, 1994.
Uebersax, L., H. Hagenmüller, S. Hofmann, E. Gruenblatt, R. Müller, G. Vunjaknovakovic, D. L. Kaplan, H. Merkle, and L. Meinel. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 12(12):3417–3429, 2006.
van Lenthe, G. H., H. Hagenmüller, M. Bohner, S. J. Hollister, L. Meinel, and R. Müller. Nondestructive micro-computed tomography for biological imaging and quantification of scaffold–bone interaction in vivo. Biomaterials 28(15):2479–2490, 2007.
Van Ransbeeck, P., F. Maes, S. Impens, H. Van Oosterwyck, and P. Verdonck. Numerical modeling of perfusion flow in irregular scaffolds. In: 4th European Conference of the International Federation for Medical and Biological Engineering, 2009, pp. 2677–2680.
Vepari, C., and D. L. Kaplan. Silk as a biomaterial. Prog. Polym. Sci. 32(8):991–1007, 2007.
Vetsch, J. R., S. Hofmann, and R. Müller. Increased flow velocity positively influences osteogenic differentiation of human stem cells in a perfusion bioreactor. In: 3rd Termis World Congress, 2012.
Vetsch, J. R., R. Müller, and S. Hofmann. The evolution of simulation techniques for dynamic bone tissue engineering in bioreactors. J. Tissue Eng. Regen. Med., 2013. doi: 10.1002/term.1733.
Voronov, R., S. VanGordon, V. I. Sikavitsas, and D. V. Papavassiliou. Computational modeling of flow-induced shear stresses within 3D salt-leached porous scaffolds imaged via micro-CT. J. Biomech. 43(7):1279–1286, 2010.
Yao, Y., Y. Yu, J. Guo, Y. Qian, and J. Wang. Prediction of shear stress distribution throughout 3D bone scaffold in perfusion environment. In: 2010 International Conference on Image Analysis and Signal Processing (IASP), 2010, pp. 51–54.
Zermatten, E., S. Haussener, M. Schneebeli, and A. Steinfeld. Tomography-based determination of permeability and Dupuit-Forchheimer coefficient of characteristic snow samples. J. Glaciol. 57(205):811–816, 2011.
Acknowledgments
The authors gratefully acknowledge financial support from the European Union (BIODESIGN FP7-NMP-2010-LARGE-4). We thank H. Fries and A. Haselbacher for their support with the computational grid generation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott I Simon oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Zermatten, E., Vetsch, J.R., Ruffoni, D. et al. Micro-Computed Tomography Based Computational Fluid Dynamics for the Determination of Shear Stresses in Scaffolds Within a Perfusion Bioreactor. Ann Biomed Eng 42, 1085–1094 (2014). https://doi.org/10.1007/s10439-014-0981-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-0981-0