Abstract
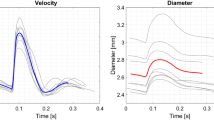
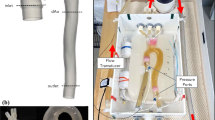
The purpose of this study is to investigate how the imposition of personalized, non-invasively measured blood flow rates as boundary conditions (BCs) influences image-based computational hemodynamic studies in the human aorta. We extracted from 4D phase-contrast MRI acquisitions of a healthy human (1) the geometry of the thoracic aorta with supra-aortic arteries and (2) flow rate waveforms at all boundaries. Flow simulations were carried out, and the implications that the imposition of different BC schemes based on the measured flow rates have on wall shear stress (WSS)-based indicators of abnormal flow were analyzed. Our results show that both the flow rate repartition among the multiple outlets of the aorta and the distribution and magnitude of the WSS-based indicators are strongly influenced by the adopted BC strategy. Keeping as reference hemodynamic model the one where the applied BC scheme allowed to obtain a satisfactory agreement between the computed and the measured flow rate waveforms, differences in WSS-based indicators up to 49% were observed when the other BC strategies were applied. In conclusion, we demonstrate that in subject-specific computational hemodynamics models of the human aorta the imposition of BC settings based on non-invasively measured flow rate waveforms influences indicators of abnormal flow to a large extent. Hence, a BCs set-up assuring realistic, subject-specific instantaneous flow rate distribution must be applied when BCs such as flow rates are prescribed.







Similar content being viewed by others
Abbreviations
- AAo:
-
Ascending aorta
- BC:
-
Boundary condition
- BCA:
-
Brachiocephalic artery
- CFD:
-
Computational fluid dynamics
- LCCA:
-
Left common carotid artery
- LSA:
-
Left subclavian artery
- OSI:
-
Oscillatory shear index
- PC-MRI:
-
Phase-contrast magnetic resonance imaging
- RRT:
-
Relative residence time
- TAWSS:
-
Time-averaged wall shear stress
- WSS:
-
Wall shear stress
References
Antiga, L., M. Piccinelli, L. Botti, B. Ene-Iordache, A. Remuzzi, and D. A. Steinman. An image-based modelling framework for patient-specific computational haemodynamics. Med. Biol. Eng. Comput. 46(11):1097–1112, 2008.
Augst, A. D., D. C. Barratt, A. D. Hughes, S. A. Thom, and X. Y. Xu. Various issues relating to computational fluid dynamics simulations of carotid bifurcation flow based on models reconstructed from three-dimensional ultrasound images. Proc. Inst. Mech. Eng. H 217(5):393–403, 2003.
Barakat, A. I., T. Karino, and C. K. Colton. Microcinematographic studies of flow patterns in the excised rabbit aorta and its major branches. Biorheology 34(3):195–221, 1997.
Boussel, L., V. Rayz, G. Acevedo-Bolton, M. T. Lawton, R. Higashida, W. S. Smith, W. L. Young, and D. Saloner. Phase-contrast magnetic resonance imaging measurements in intracranial aneurysms in vivo of flow patterns, velocity fields, and wall shear stress: comparison with computational fluid dynamics. Magn. Reson. Med. 61(2):409–417, 2009.
Cebral, J. R., M. A. Castro, S. Appanaboyina, C. M. Putman, D. Millan, and A. F. Frangi. Efficient pipeline for image-based patient-specific analysis of cerebral aneurysm hemodynamics: technique and sensitivity. IEEE Trans. Med. Imaging 24(4):457–467, 2004.
De Santis, G., M. De Beule, P. Segers, P. Verdonck, and B. Verhegghe. Patient-specific computational haemodynamics: generation of structured and conformal hexahedral meshes from triangulated surfaces of vascular bifurcations. Comput. Methods Biomech. Biomed. Eng. doi:10.1080/10255842.2010.495066, 2011.
De Santis, G., P. Mortier, M. De Beule, P. Segers, P. Verdonck, and B. Verhegghe. Patient-specific computational fluid dynamics: structured mesh generation from coronary angiography. Med. Biol. Eng. Comput. 48(4):371–380, 2010.
Del Gaudio, C., U. Morbiducci, and M. Grigioni. Time dependent non-newtonian numerical study of the flow field in a realistic model of the aortic arch. Int. J. Artif. Organs. 29(7):709–718, 2006.
Formaggia, L., J. F. Gerbeau, F. Nobile, and A. Quarteroni. On the coupling of 3D and 1D navier-stokes equations for flow problems in compliant vessels. Comput. Methods Appl. Mech. Eng. 191(6-7):561–582, 2001.
Formaggia, L., J. F. Gerbeau, F. Nobile, and A. Quarteroni. Numerical treatment of defective boundary conditions for the Navier–Stokes equation. SIAM J. Numer. Anal. 40:376–401, 2002.
Formaggia, L., A. Veneziani, and C. Vergara. A new approach to numerical solution of defective boundary value problems in incompressible fluid dynamics. SIAM J. Numer. Anal. 46(6):2769–2794, 2008.
Grinberg, L., and G. E. M. Karniadakis. Outflow boundary conditions for arterial networks with multiple outlets. Ann. Biomed. Eng. 36(9):1496–1514, 2008.
Groen, H. C., L. Simons, Q. J. van den Bouwhuijsen, E. M. Bosboom, F. J. Gijsen, A. G. van der Giessen, F. N. van de Fosse, A. Hofman, A. F. van der Steen, J. C. Witteman, A. van der Lugt, and J. J. Wentzel. MRI-based quantification of outflow boundary conditions for computational fluid dynamics of stenosed human carotid arteries. J. Biomech. 43(12):2332–2338, 2010.
He, X., and D. N. Ku. Pulsatile flow in the human left coronary artery bifurcation: average conditions. ASME J. Biomech. Eng. 118(1):74–82, 1996.
Heywood, J., R. Rannacher, and S. Turek. Artificial boundary and flux and pressure conditions for the incompressible Navier-Stokes equations. Int. J. Numer. Methods Fluids 22:325–352, 1996.
Himburg, H. A., D. M. Grzybowski, A. Hazel, J. A. LaMack, X. M. Li, and M. H. Friedman. Spatial comparison between wall shear stress measures and porcine arterial endothelial permeability. Am. J. Physiol. Heart Circ. Physiol. 286(5):H1916–H1922, 2004.
Kim, H. J., C. A. Figueroa, T. J. R. Hughes, K. E. Jansen, and C. A. Taylor. Augmented Lagrangian method for constraining the shape of velocity profiles at outlet boundaries for three-dimensional finite element simulations of blood flow. Comput. Methods Appl. Mech. Eng. 198:3551–3556, 2009.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low and oscillating shear stress. Arteriosclerosis 5(3):293–302, 1985.
Lee, S. W., L. Antiga, J. D. Spence, and D. A. Steinman. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39(8):2341–2347, 2008.
Lee, S. W., L. Antiga, and D. A. Steinman. Correlations among indicators of disturbed flow at the normal carotid bifurcation. ASME J. Biomech. Eng. 131(6):061013, 7 pp, 2009.
Liu, X., Y. Fan, and X. Deng. Effect of spiral flow on the transport of oxygen in the aorta: a numerical study. Ann. Biomed. Eng. 38(3):917–926, 2009.
Liu, X., Y. Fan, X. Deng, and F. Zhan. Effect of non-newtonian and pulsatile blood flow on mass transport in the human aorta. J. Biomech. 44(6):1123–1131, 2011.
Liu, X., F. Pu, Y. Fan, X. Deng, D. Li, and S. Li. A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. Am. J. Physiol. Heart Circ. Physiol. 297:H163–H170, 2009.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Moore, J. E., C. Xu, S. Glagov, C. K. Zarins, and D. N. Ku. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis 110(2):225–240, 1994.
Morbiducci, U., D. Gallo, D. Massai, R. Ponzini, M. A. Deriu, L. Antiga, A. Redaelli, and F. M. Montevecchi. On the importance of blood rheology for bulk flow in hemodynamic models of the carotid bifurcation. J. Biomech. 44:2427–2438, 2011.
Morbiducci, U., D. Gallo, R. Ponzini, D. Massai, L. Antiga, A. Redaelli, and F. M. Montevecchi. Quantitative analysis of bulk flow in image-based haemodynamic models of the carotid bifurcation: the influence of outflow conditions as test case. Ann. Biomed. Eng. 38(12):3688–3705, 2010.
Morbiducci, U., R. Ponzini, G. Rizzo, M. Cadioli, A. Esposito, F. M. Montevecchi, and A. Redaelli. Mechanistic insight into the physiological relevance of helical blood flow in the human aorta: an in vivo study. Biomech. Model. Mechanobiol. 10:339–355, 2011.
Morbiducci, U., D. Gallo, D. Massai, F. Consolo, R. Ponzini, L. Antiga, C. Bignardi, M. A. Deriu, and A. Redaelli. Outflow conditions for image-based haemodynamic models of the carotid bifurcation. Implications for indicators of abnormal flow. J. Biomech. Eng. 132:091005, 11 pp, 2010.
Morbiducci, U., R. Ponzini, G. Rizzo, M. Cadioli, A. Esposito, F. De Cobelli, A. Del Maschio, F. M. Montevecchi, and A. Redaelli. In vivo quantification of helical blood flow in human aorta by time-resolved three-dimensional cine phase contrast MRI. Ann. Biomed. Eng. 37:516–531, 2009.
Olufsen, M. S. Structured tree outflow condition for blood flow in larger systemic arteries. Am. J. Physiol. 276:H257–H268, 1999.
Ponzini, R., M. Lemma, U. Morbiducci, F. M. Montevecchi, and A. Redaelli. Doppler derived quantitative flow estimate in coronary artery bypass graft: a computational multi-scale model for the evaluation of the current theory. Med. Eng. Phys. 30(7):809–816, 2008.
Quarteroni, A., S. Ragni, and A. Veneziani. Coupling between lumped and distributed models for blood flow problems. Comput. Visual. Sci. 4:111–124, 2001.
Shahcheraghi, N., H. A. Dwyer, A. Y. Cheer, A. I. Barakat, and T. Rutaganira. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J. Biomech. Eng. 124:378–388, 2002.
Spilker, R. L., and C. A. Taylor. Tuning multidomain hemodynamic simulations to match physiological measurements. Ann. Biomed. Eng. 38(8):2635–2648, 2010.
Tan, F. P. P., A. Borghi, R. H. Mohiaddin, N. B. Wood, S. Thom, and X. Y. Xu. Analysis of flow patterns in a patient-specific thoracic aneurysm model. Comput. Struct. 87:680–690, 2009.
Taylor, C. A., C. P. Cheng, L. A. Espinosa, B. T. Tang, D. Parker, and R. J. Herfkens. In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise. Ann. Biomed. Eng. 30:402–408, 2002.
Trachet, B., M. Renard, G. De Santis, S. Staelens, J. De Backer, L. Antiga, B. Loeys, and P. Segers. An integrated framework to quantitatively link mouse-specific hemodynamics to aneurysm formation in angiotensin II—infused ApoE−/− mice. Ann. Biomed. Eng. doi:10.1007/s10439-011-0330-5, 2011.
Van der Giessen, A. G., H. C. Groen, P. A. Doriot, P. J. de Feyter, A. F. van der Steen, F. N. van de Fosse, J. J. Wentzel, and F. J. Gijsen. The influence of boundary conditions on wall shear stress distribution in patient specific coronary trees. J. Biomech. 44(6):1089–1095, 2011.
Veneziani, A., and C. Vergara. Flow rate defective boundary conditions in haemodynamics simulations. Int. J. Numer. Methods Fluids 47(8–9):803–816, 2005.
Vignon-Clementel, I. E., C. A. Figueroa, K. E. Jansenc, and C. A. Taylor. Outflow boundary conditions for three dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195:3776–3796, 2006.
Wen, C., A. Yang, L. Tseng, and J. Chai. Investigation of pulsatile flowfield in healthy thoracic aorta models. Ann. Biomed. Eng. 38(2):391–402, 2010.
Westerhof, N., J. W. Lankhaar, and B. E. Westerhof. The arterial windkessel. Med. Biol. Eng. Comput. 47(2):131–141, 2009.
White, F. M. Viscous Fluid Flow. New York: McGraw-Hill, 1979.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Kerry Hourigan oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gallo, D., De Santis, G., Negri, F. et al. On the Use of In Vivo Measured Flow Rates as Boundary Conditions for Image-Based Hemodynamic Models of the Human Aorta: Implications for Indicators of Abnormal Flow. Ann Biomed Eng 40, 729–741 (2012). https://doi.org/10.1007/s10439-011-0431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0431-1