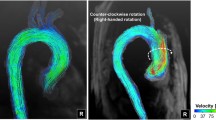
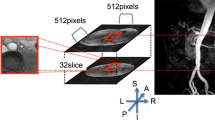
Abstract
To test the hypothesis that the spiral flow pattern in the human aorta may play a beneficial role in the transport of oxygen, hence sparing the ascending aorta from atherosclerosis, a comparative study on the distribution of oxygen flux to the arterial wall was carried out numerically for three aorta models. The first one (Model 1) was constructed based on MRI images of a human aorta acquired in vivo. The second (Model 2) was made the same as Model 1 but without the three branches. The third (Model 3) was similar to Model 2 only with the aortic torsion removed. The results showed that without the torsion, the flow fashion in Model 3 was very different from the swirling flows in Models 1 and 2 and exhibited the typical characteristics of Dean flow with two symmetrical helical structures. In Models 1 and 2, the average Sherwood number (Sh) in the ascending aorta with the presence of a strong spiral flow was not only higher than that in the inner wall of the descending aorta where the spiral flow faded out, but also generally higher than that in Model 3. The comparison between Models 2 and 3 demonstrated that the averaged Sh from ascending aorta to aortic arch was relatively higher for Model 2. In conclusion, the spiral or swirling flow may have certain physiological significance in the aorta and play a positive role in the transport of oxygen by enhancing oxygen flux to the arterial wall.






Similar content being viewed by others
References
Back, L. H., J. R. Radbill, and D. W. Crawford. Analysis of oxygen transport from pulsatile viscous blood flow to diseased coronary arteries of man. J. Biomech. 10:763–774, 1977.
Berceli, S. A., V. S. Warty, R. A. Sheppeck, W. A. Mandarino, S. K. Tanksale, and H. S. Borovetz. Hemodynamics and low density lipoprotein metabolism. Rates of low density lipoprotein incorporation and degradation along medial and lateral walls of the rabbit aorto-iliac bifurcation. Arteriosclerosis 10:686–694, 1990.
Bogren, H. G., and M. H. Buonocore. 4D magnetic resonance velocity mapping of blood flow patterns in the aorta in young vs. elderly normal subjects. J. Magn. Reson. Imaging 10:861–869, 1999.
Buerk, D. G., and T. K. Goldstick. Arterial wall oxygen consumption rate varies spatially. Am. J. Physiol. 243:H948–H959, 1982.
Caro, C. G., D. J. Doorly, M. Tarnawski, K. T. Scott, Q. Long, and C. L. Dumoulin. Non-planar curvature and branching of arteries and non-planar-type flow. Proc. Roy. Soc. Lond. A 452:185–197, 1996.
Caro, C. G., T. J. Pedley, R. C. Schroter, and W. A. Seed. The Mechanics of the Circulation. Oxford: Oxford University Press, 1978.
Coppola, G., and C. Caro. Oxygen mass transfer in a model three-dimensional artery. J. R. Soc. Interface 5:1067–1075, 2008.
Deng, X. Y., Y. Marois, T. How, Y. Merhi, M. King, R. Guidoin, and T. Karino. Luminal surface concentration of lipoprotein (LDL) and its effect on the wall uptake of cholesterol by canine carotid arteries. J. Vasc. Surg. 21:135–145, 1995.
Friedman, M. H. Geometric risk factors for arteriosclerosis. Johns Hopkins APL Technical Digest (Appl. Phys. Lab.) 4:85–95, 1983.
Friedman, M. H., C. B. Bargeron, D. D. Duncan, G. M. Hutchins, and F. F. Mark. Effects of arterial compliance and non-Newtonian rheology on correlations between intimal thickness and wall shear. J. Biomech. Eng. 114:317–320, 1992.
Fung, Y. C. Biomechanics: Circulation (2nd ed.). New York: Springer, p. 108, 1997.
Gould, S. E. Pathology of the Heart and Blood Vessels. Illinois: Charles & Thomas, pp. 953–960, 1968.
Hope, T. A., M. Markl, L. Wigström, M. T. Alley, D. C. Miller, and R. J. Herfkens. Comparison of flow patterns in ascending aortic aneurysms and volunteers using four-dimensional magnetic resonance velocity mapping. J. Magn. Reson. Imaging 26:1471–1479, 2007.
Houston, J. G., S. J. Gandy, D. G. Sheppard, J. B. Dick, J. J. Belch, and P. A. Stonebridge. Two-dimensional flow quantitative MRI of aortic arch blood flow patterns: effect of age, sex, and presence of carotid atheromatous disease on prevalence of spiral blood flow. J. Magn. Reson. Imaging 18:169–174, 2003.
Hueper, W. C. Arteriosclerosis. Arch. Path. 39:162–182, 1945.
Hultén, L. M., and M. Levin. The role of hypoxia in atherosclerosis. Curr. Opin. Lipidol. 20:409–414, 2009.
Jin, S., J. Oshinski, and D. P. Giddens. Effects of wall motion and compliance on flow patterns in the ascending aorta. J. Biomech. Eng. 125:347–354, 2003.
Karino, T., T. Asakura, and S. Mabuchi. Role of hemodynamic factors in atherogenesis. Adv. Exp. Med. Biol. 242:51–57, 1988.
Kilner, P. J., G. Z. Yang, R. H. Mohiaddin, D. N. Firmin, and D. B. Longmore. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation 88:2235–2247, 1993.
Kjeldsen, K., P. Astrup, and J. Wanstrup. Reversal of rabbit atheromatosis by hyperoxia. J. Atheroscler. Res. 10:173–178, 1969.
Kjeldsen, K., J. Wanstrup, and P. Astrup. Enhancing influence of arterial hypoxia on the development of atheromatosis in cholesterol-fed rabbits. J. Atheroscler. Res. 8:835–845, 1968.
Kolandavel, M. K., E. T. Fruend, S. Ringgaard, and P. G. Walker. The effects of time varying curvature on species transport in coronary arteries. Ann. Biomed. Eng. 34:1820–1832, 2006.
Ku, D. N. Blood flow in arteries. Annu. Rev. Fluid Mech. 29:399–434, 1997.
Lattimore, J. D., I. Wilcox, S. Nakhla, M. Langenfeld, W. Jessup, and D. S. Celermajer. Repetitive hypoxia increases lipid loading in human macrophages—a potentially atherogenic effect. Atherosclerosis 179:255–259, 2005.
Levy, Y., D. Degani, and A. Seginer. Graphical visualization of vortical flows by means of helicity. AIAA J. 28:1347–1352, 1990.
Liu, X., F. Pu, Y. B. Fan, X. Y. Deng, D. Y. Li, and S. Y. Li. A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. Am. J. Physiol. Heart Circ. Physiol. 297:H163–H170, 2009.
Ma, P., X. Li, and D. N. Ku. Heat and mass transfer in a separated flow region for high Prandtl and Schmidt numbers under pulsatile flow conditions. Int. J. Heat Mass Transfer 37:2723–2736, 1994.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Matsushita, H., R. Morishita, T. Nata, M. Aoki, H. Nakagami, Y. Taniyama, K. Yamamoto, J. Higaki, K. Yasufumi, and T. Ogihara. Hypoxia-induced endothelial apoptosis through nuclear factor-kappaB (NF-kappaB)-mediated bcl-2 suppression: in vivo evidence of the importance of NF-kappaB in endothelial cell regulation. Circ. Res. 12:974–981, 2000.
Moffatt, H. K. The degree of knottedness of tangled vortex lines. J. Fluid Mech. 36:17–29, 1969.
Moore, J. A., and C. R. Ethier. Oxygen mass transfer calculations in large arteries. J. Biomech. Eng. 119:469–475, 1997.
Moore, J. E., S. E. Maiser, Jr., D. N. Ku, and P. Boesiger. Hemodynamics in the abdominal aorta: a comparison of in vitro and in vivo measurements. J. Appl. Physiol. 76:1520–1527, 1994.
Morbiducci, U., R. Ponzini, M. Grigioni, and A. Redaelli. Helical flow as fluid dynamic signature for atherogenesis risk in aortocoronary bypass. A numeric study. J. Biomech. 40:519–534, 2007.
Nerem, R. M. Vascular fluid mechanics, the arterial wall, and atherosclerosis. J. Biomech. Eng. 114:274–282, 1992.
O’Callaghan, S., M. Walsh, and T. McGloughlin. Numerical modelling of Newtonian and non-Newtonian representation of blood in a distal end-to-side vascular bypass graft anastomosis. Med. Eng. Phys. 28:70–74, 2006.
Ogawa, S., H. Gerlach, C. Esposito, A. Pasagian-Macaulay, J. Brett, and D. Stern. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J. Clin. Invest. 85:1090–1098, 1990.
Okamoto, R., M. Hatani, M. Tsukitani, A. Suehiro, M. Fujino, N. Imai, S. Takano, Y. Watanabe, and H. Fukuzaki. The effect of oxygen on the development of atherosclerosis in WHHL rabbits. Atherosclerosis 47:47–53, 1983.
Qiu, Y., and J. M. Tarbell. Numerical simulation of oxygen mass transfer in a compliant curved tube model of a coronary artery. Ann. Biomed. Eng. 28:26–38, 2000.
Rodkiewicz, C. M. Localization of early atherosclerotic lesions in the aortic arch in the light of fluid flow. J. Biomech. 8:149–156, 1975.
Rydberg, E. K., A. Krettek, C. Ullström, K. Ekström, P. A. Svensson, L. M. Carlsson, A. C. Jönsson-Rylander, G. I. Hansson, W. McPheat, O. Wiklund, B. G. Ohlsson, and L. M. Hultén. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arterioscler. Thromb. Vasc. Biol. 24:2040–2045, 2004.
Sabbah, H. N., F. Khaja, J. F. Brymer, E. T. Hawkins, and P. D. Stein. Blood velocity in the right coronary: relation to the distribution of atherosclerotic lesions. Am. J. Cardiol. 53:1008–1012, 1984.
Santilli, S. M., R. B. Stevens, J. G. Anderson, W. D. Payne, and M. D. Caldwell. Transarterial wall oxygen gradients at the dog carotid bifurcation. Am. J. Physiol. 268:H155–H161, 1995.
Schneiderman, G., and T. K. Goldstick. Significance of luminal plasma layer resistance in arterial wall oxygen supply. Atherosclerosis 31:11–20, 1978.
Schneiderman, G., L. F. Mockros, and T. K. Goldstick. Effect of pulsatility on oxygen transport to the human arterial wall. J. Biomech. 15:849–858, 1982.
Seed, W. A., and N. B. Wood. Velocity patterns in the aorta. Cardiovasc. Res. 5:319–330, 1971.
Segadal, L., and K. Matre. Blood velocity distribution in the human ascending aorta. Circulation 76:90–100, 1987.
Shahcheraghi, N., H. A. Dwyer, A. Y. Cheer, A. I. Barakat, and T. Rutaganira. Unsteady and three-dimensional simulation of blood flow in the human aortic arch. J. Biomech. Eng. 124:378–387, 2002.
Sluimer, J. C., J. M. Gasc, J. L. van Wanroij, N. Kisters, M. Groeneweg, M. D. Sollewijn Gelpke, J. P. Cleutjens, L. H. van den Akker, P. Corvol, B. G. Wouters, M. J. Daemen, and A. P. Bijnens. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J. Am. Coll. Cardiol. 51:1258–1265, 2008.
Stein, T. R., J. C. Martin, and K. H. Keller. Steady-state oxygen transport through red blood cell suspensions. J. Appl. Physiol. 31:397–402, 1971.
Tada, S., and J. M. Tarbell. Oxygen mass transport in a compliant carotid bifurcation model. Ann. Biomed. Eng. 34:1389–1399, 2006.
Tarbell, J. M. Mass transport in arteries and the localization of atherosclerosis. Annu. Rev. Biomed. Eng. 5:79–118, 2003.
Yashiro, K., H. Shiratori, and H. Hamada. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450:285–288, 2007.
Acknowledgments
This work was supported by Grants-in-Aid from the National Natural Science Foundation of China (No. 10632010, 30670517).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Gerald Saidel oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Liu, X., Fan, Y. & Deng, X. Effect of Spiral Flow on the Transport of Oxygen in the Aorta: A Numerical Study. Ann Biomed Eng 38, 917–926 (2010). https://doi.org/10.1007/s10439-009-9878-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-009-9878-8