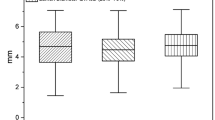
Abstract
Vessel geometry determines blood flow dynamics and plays a crucial role in the pathogenesis of vascular disease. In vivo assessment of three-dimensional (3D) vessel anatomy is vital to improve the realism of arterial flow model geometries and investigate factors associated with the localisation of atherosclerosis. The quantification of vascular geometry is also particularly important for the proper design and preclinical testing of endovascular devices used to treat peripheral arterial disease. The purpose of this study was to quantitatively evaluate the intersubject variability of 3D branching and curvature of the abdominal aorta and its major peripheral arteries. Contrast-enhanced renal MRA scans of healthy abdominal vessels obtained in 12 subjects (8 men, 4 women mean age 49 years, range 27–84 years) were segmented, and smoothed centerlines were determined as descriptors of arterial geometry. Robust techniques were employed to characterise non-planar vessel curvature, arterial taper, and 3D branching parameters. Noticeable 3D curvature and tapering were quantified for the proximal anterior visceral and renal branches. Mean 3D branching angles of 63.5 ± 10.1° and 73.1 ± 6.8° were established for the right and left renal arteries, respectively. Angles describing the ostial position and initial trajectory of the renal arteries confirmed the antero-lateral origin and direction of the right and the more lateral orientation of the left. The anterior visceral branches emerged predominantly from the left side of the anterior aortic wall. Branching parameters determined at the aortic bifurcation demonstrated mild asymmetry and non-planarity at this location. In summary, the results from this study address the scarcity of available in vivo 3D quantitative geometric data relating to the abdominal vasculature and reflect the geometric variability in living subjects.









Similar content being viewed by others
References
Ajmani, M. L., and K. Ajmani. To study the intrarenal vascular segments of human kidney by corrosion cast technique. Anat. Anz. 154:293–303, 1983.
Antiga, L., and D. A. Steinman. Robust and objective decomposition and mapping of bifurcating vessels. IEEE Trans. Med. Imaging 23:704–713, 2004.
Aubert, J., and K. Koumare. Variations of origin of the renal artery: a review covering 403 aortographies. Eur. Urol. 1:182–188, 1975.
Aytac, S. K., H. Yigit, T. Sancak, and H. Ozcan. Correlation between the diameter of the main renal artery and the presence of an accessory renal artery: sonographic and angiographic evaluation. J. Ultrasound Med. 22:433–439, 2003.
Baden, J. G., D. J. Racy, and T. M. Grist. Contrast-enhanced three-dimensional magnetic resonance angiography of the mesenteric vasculature. J. Magn. Reson. Imaging 10:369–375, 1999.
Bargeron, C. B., G. M. Hutchins, G. W. Moore, O. J. Deters, F. F. Mark, and M. H. Friedman. Distribution of the geometric parameters of human aortic bifurcations. Arteriosclerosis 6:109–113, 1986.
Baumgartner, I., K. von Aesch, D. D. Do, J. Triller, M. Birrer, and F. Mahler. Stent placement in ostial and nonostial atherosclerotic renal arterial stenoses: a prospective follow-up study. Radiology 216:498–505, 2000.
Bekkers, E. J., and C. A. Taylor. Multiscale vascular surface model generation from medical imaging data using hierarchical features. IEEE Trans. Med. Imaging 27:331–341, 2008.
Beregi, J. P., B. Mauroy, S. Willoteaux, C. Mounier-Vehier, M. Remy-Jardin, and J. Francke. Anatomic variation in the origin of the main renal arteries: spiral CTA evaluation. Eur. Radiol. 9:1330–1334, 1999.
Caro, C. G., D. J. Doorly, M. Tarnawski, K. T. Scott, Q. Long, and C. L. Dumoulin. Non-planar curvature and branching of arteries and non-planar-type flow. Proc. R. Soc. Lond. 452:185–197, 1996.
Cebral, J. R., and R. Lohner. From medical images to anatomically accurate finite element grids. Int. J. Numer. Methods Eng. 51:985–1008, 2001.
Choi, G., C. P. Cheng, N. M. Wilson, and C. A. Taylor. Methods for quantifying three-dimensional deformation of arteries due to pulsatile and nonpulsatile forces: implications for the design of stents and stent grafts. Ann. Biomed. Eng. 37:14–33, 2009.
Choi, G., L. K. Shin, C. A. Taylor, and C. P. Cheng. In vivo deformation of the human abdominal aorta and common iliac arteries with hip and knee flexion: implications for the design of stent-grafts. J. Endovasc. Ther. 16:531–538, 2009.
Cornhill, J. F., E. E. Herderick, and H. C. Stary. Topography of human aortic sudanophilic lesions. Monogr. Atheroscler. 15:13–19, 1990.
DeBakey, M. E., G. M. Lawrie, and D. H. Glaeser. Patterns of atherosclerosis and their surgical significance. Ann. Surg. 201:115–131, 1985.
Draney, M. T., C. K. Zarins, and C. A. Taylor. Three-dimensional analysis of renal artery bending motion during respiration. J. Endovasc. Ther. 12:380–386, 2005.
Fanucci, E., A. Orlacchio, and M. Pocek. The vascular geometry of human arterial bifurcations. Invest. Radiol. 23:713–718, 1988.
Fleischmann, D., T. J. Hastie, F. C. Dannegger, D. S. Paik, M. Tillich, C. K. Zarins, and G. D. Rubin. Quantitative determination of age-related geometric changes in the normal abdominal aorta. J. Vasc. Surg. 33:97–105, 2001.
Friedman, M. H., O. J. Deters, F. F. Mark, C. B. Bargeron, and G. M. Hutchins. Arterial geometry affects hemodynamics. A potential risk factor for athersoclerosis. Atherosclerosis 46:225–231, 1983.
Garcier, J. M., B. De Fraissinette, M. Filaire, P. Gayard, T. Therre, A. Ravel, and L. Boyer. Origin and initial course of the renal arteries: a radiological study. Surg. Radiol. Anat. 23:51–55, 2001.
Geboes, K., K. P. Geboes, and G. Maleux. Vascular anatomy of the gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 15:1–14, 2001.
Glagov, S., D. A. Rowley, and R. I. Kohut. Atherosclerosis of human aorta and its coronary and renal arteries. A consideration of some hemodynamic factors which may be related to the marked differences in atherosclerotic involvement of the coronary and renal arteries. Arch. Pathol. 72:558–571, 1961.
Hirsch, A. T., Z. J. Haskal, N. R. Hertzer, C. W. Bakal, M. A. Creager, J. L. Halperin, L. F. Hiratzka, W. R. Murphy, J. W. Olin, J. B. Puschett, K. A. Rosenfield, D. Sacks, J. C. Stanley, L. M. Taylor, Jr., C. J. White, J. White, R. A. White, E. M. Antman, S. C. Smith, Jr., C. D. Adams, J. L. Anderson, D. P. Faxon, V. Fuster, R. J. Gibbons, S. A. Hunt, A. K. Jacobs, R. Nishimura, J. P. Ornato, R. L. Page, and B. Riegel. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 113:e463–e654, 2006.
Horejs, D., P. M. Gilbert, S. Burstein, and R. L. Vogelzang. Normal aortoiliac diameters by CT. J. Comput. Assist. Tomogr. 12:602–603, 1988.
Jeays, A. D., P. V. Lawford, R. Gillott, P. Spencer, D. C. Barber, K. D. Bardhan, and D. R. Hose. Characterisation of the haemodynamics of the superior mesenteric artery. J. Biomech. 40:1916–1926, 2007.
Kaatee, R., F. J. Beek, E. J. Verschuyl, P. J. vd Ven, J. J. Beutler, J. P. van Schaik, and W. P. Mali. Atherosclerotic renal artery stenosis: ostial or truncal? Radiology 199:637–640, 1996.
Kahraman, H., M. Ozaydin, E. Varol, S. M. Aslan, A. Dogan, A. Altinbas, M. Demir, O. Gedikli, G. Acar, and O. Ergene. The diameters of the aorta and its major branches in patients with isolated coronary artery ectasia. Tex. Heart Inst. J. 33:463–468, 2006.
Kilner, P. J., G. Z. Yang, R. H. Mohiaddin, D. N. Firmin, and D. B. Longmore. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation 88:2235–2247, 1993.
Kosinski, H. Variability of places of origin of the human renal arteries. Folia Morphol. (Warsz) 53:111–116, 1994.
Ku, D. N., S. Glagov, J. E. Moore, Jr., and C. K. Zarins. Flow patterns in the abdominal aorta under simulated postprandial and exercise conditions: an experimental study. J. Vasc. Surg. 9:309–316, 1989.
Ladak, H. M., J. S. Milner, and D. A. Steinman. Rapid three-dimensional segmentation of the carotid bifurcation from serial MR images. J. Biomech. Eng. 122:96–99, 2000.
Lederle, F. A., G. R. Johnson, S. E. Wilson, I. L. Gordon, E. P. Chute, F. N. Littooy, W. C. Krupski, D. Bandyk, G. W. Barone, L. M. Graham, R. J. Hye, and D. B. Reinke. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Investigators. J. Vasc. Surg. 26:595–601, 1997.
Lee, D., and J. Y. Chen. Numerical simulation of steady flow fields in a model of abdominal aorta with its peripheral branches. J. Biomech. 35:1115–1122, 2002.
Lee, Y. T., W. F. Keitzer, F. R. Watson, and H. Liu. Vascular geometry at the abdominal aortic bifurcation. J. Am. Med. Women Assoc. 37:77–81, 1982.
Liepsch, D., A. Poll, J. Strigberger, H. N. Sabbah, and P. D. Stein. Flow visualization studies in a mold of the normal human aorta and renal arteries. J. Biomech. Eng. 111:222–227, 1989.
Lilly, M. P., T. R. Harward, W. R. Flinn, D. R. Blackburn, P. M. Astleford, and J. S. Yao. Duplex ultrasound measurement of changes in mesenteric flow velocity with pharmacologic and physiologic alteration of intestinal blood flow in man. J. Vasc. Surg. 9:18–25, 1989.
Long, Q., X. Y. Xu, M. Bourne, and T. M. Griffith. Numerical study of blood flow in an anatomically realistic aorto-iliac bifurcation generated from MRI data. Magn. Reson. Med. 43:565–576, 2000.
Longia, G. S., V. Kumar, and C. D. Gupta. Intrarenal arterial pattern of human kidney-corrosion cast study. Anat. Anz. 155:183–194, 1984.
MacLean, N. F., and M. R. Roach. Thickness, taper, and ellipticity in the aortoiliac bifurcation of patients aged 1 day to 76 years. Heart Vessels 13:95–101, 1998.
Moore, Jr., J., and J. L. Berry. Fluid and solid mechanical implications of vascular stenting. Ann. Biomed. Eng. 30:498–508, 2002.
Moore, Jr., J. E., and D. N. Ku. Pulsatile velocity measurements in a model of the human abdominal aorta under resting conditions. J. Biomech. Eng. 116:337–346, 1994.
Moore, Jr., J. E., and D. N. Ku. Pulsatile velocity measurements in a model of the human abdominal aorta under simulated exercise and postprandial conditions. J. Biomech. Eng. 116:107–111, 1994.
Moore, Jr., J. E., D. N. Ku, C. K. Zarins, and S. Glagov. Pulsatile flow visualization in the abdominal aorta under differing physiologic conditions: implications for increased susceptibility to atherosclerosis. J. Biomech. Eng. 114:391–397, 1992.
Moreno, M. R., J. E. Moore, Jr., and R. Meuli. Cross-sectional deformation of the aorta as measured with magnetic resonance imaging. J. Biomech. Eng. 120:18–21, 1998.
Nguyen, N. D., and A. K. Haque. Effect of hemodynamic factors on atherosclerosis in the abdominal aorta. Atherosclerosis 84:33–39, 1990.
O’Flynn, P. M., G. O’Sullivan, and A. S. Pandit. Methods for three-dimensional geometric characterization of the arterial vasculature. Ann. Biomed. Eng. 35:1368–1381, 2007.
Ozan, H., A. Alemdaroglu, A. Sinav, and Y. Gumusalan. Location of the ostia of the renal arteries in the aorta. Surg. Radiol. Anat. 19:245–247, 1997.
Pedersen, E. M., H. W. Sung, A. C. Burlson, and A. P. Yoganathan. Two-dimensional velocity measurements in a pulsatile flow model of the normal abdominal aorta simulating different hemodynamic conditions. J. Biomech. 26:1237–1247, 1993.
Pedersen, E. M., H. W. Sung, and A. P. Yoganathan. Influence of abdominal aortic curvature and resting versus exercise conditions on velocity fields in the normal abdominal aortic bifurcation. J. Biomech. Eng. 116:347–354, 1994.
Pedersen, E. M., A. P. Yoganathan, and X. P. Lefebvre. Pulsatile flow visualization in a model of the human abdominal aorta and aortic bifurcation. J. Biomech. 25:935–944, 1992.
Pedersen, O. M., A. Aslaksen, and H. Vik-Mo. Ultrasound measurement of the luminal diameter of the abdominal aorta and iliac arteries in patients without vascular disease. J. Vasc. Surg. 17:596–601, 1993.
Pennington, N., and R. W. Soames. The anterior visceral branches of the abdominal aorta and their relationship to the renal arteries. Surg. Radiol. Anat. 27:395–403, 2005.
Perktold, K., and M. Resch. Numerical flow studies in human carotid artery bifurcations: basic discussion of the geometric factor in atherogenesis. J. Biomed. Eng. 12:111–123, 1990.
Roberts, J. C., C. Moses, and R. H. Wilkins. Autopsy studies in atherosclerosis. 1. Distribution and severity of atherosclerosis in patients dying without morphologic evidence of atherosclerotic catastrophe. Circulation 20:511–519, 1959.
Robertson, S. W., D. B. Jessup, I. J. Boero, and C. P. Cheng. Right renal artery in vivo stent fracture. J. Vasc. Interv. Radiol. 19:439–442, 2008.
Rocha-Singh, K., M. R. Jaff, and K. Rosenfield. Evaluation of the safety and effectiveness of renal artery stenting after unsuccessful balloon angioplasty: the ASPIRE-2 study. J. Am. Coll. Cardiol. 46:776–783, 2005.
Rubin, G. D., E. J. Alfrey, M. D. Dake, C. P. Semba, F. G. Sommer, P. C. Kuo, D. C. Dafoe, J. A. Waskerwitz, D. A. Bloch, and R. B. Jeffrey. Assessment of living renal donors with spiral CT. Radiology 195:457–462, 1995.
Sabbah, H. N., E. T. Hawkins, and P. D. Stein. Flow separation in the renal arteries. Arteriosclerosis 4:28–33, 1984.
Shipkowitz, T., V. G. Rodgers, L. J. Frazin, and K. B. Chandran. Numerical study on the effect of steady axial flow development in the human aorta on local shear stresses in abdominal aortic branches. J. Biomech. 31:995–1007, 1998.
Shipkowitz, T., V. G. Rodgers, L. J. Frazin, and K. B. Chandran. Numerical study on the effect of secondary flow in the human aorta on local shear stresses in abdominal aortic branches. J. Biomech. 33:717–728, 2000.
Smedby, O. Geometrical risk factors for atherosclerosis in the femoral artery: a longitudinal angiographic study. Ann. Biomed. Eng. 26:391–397, 1998.
Sonesson, B., T. Lanne, F. Hansen, and T. Sandgren. Infrarenal aortic diameter in the healthy person. Eur. J. Vasc. Surg. 8:89–95, 1994.
Sun, H., B. D. Kuban, P. Schmalbrock, and M. H. Friedman. Measurement of the geometric parameters of the aortic bifurcation from magnetic resonance images. Ann. Biomed. Eng. 22:229–239, 1994.
Suzuki, Y., F. Ikeno, J. K. Lyons, T. Koizumi, and A. C. Yeung. Novel stent system for accurate placement in aorto-ostial renal artery disease: preclinical study in porcine renal artery model. Cardiovasc. Revasc. Med. 8:99–102, 2007.
Tada, S., and J. M. Tarbell. A computational study of flow in a compliant carotid bifurcation-stress phase angle correlation with shear stress. Ann. Biomed. Eng. 33:1202–1212, 2005.
Talenfeld, A. D., R. B. Schwope, H. J. Alper, E. I. Cohen, and R. A. Lookstein. MDCT angiography of the renal arteries in patients with atherosclerotic renal artery stenosis: implications for renal artery stenting with distal protection. Am. J. Roentgenol. 188:1652–1658, 2007.
Tang, B. T., C. P. Cheng, M. T. Draney, N. M. Wilson, P. S. Tsao, R. J. Herfkens, and C. A. Taylor. Abdominal aortic hemodynamics in young healthy adults at rest and during lower limb exercise: quantification using image-based computer modeling. Am. J. Physiol. Heart Circ. Physiol. 291:H668–H676, 2006.
Tanganelli, P., G. Bianciardi, C. Simoes, V. Attino, B. Tarabochia, and G. Weber. Distribution of lipid and raised lesions in aortas of young people of different geographic origins (WHO-ISFC PBDAY Study). World Health Organization-International Society and Federation of Cardiology. Pathobiological Determinants of Atherosclerosis in Youth. Arterioscler. Thromb. 13:1700–1710, 1993.
Taylor, C. A., T. J. Hughes, and C. K. Zarins. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann. Biomed. Eng. 26:975–987, 1998.
Taylor, C. A., T. J. Hughes, and C. K. Zarins. Effect of exercise on hemodynamic conditions in the abdominal aorta. J. Vasc. Surg. 29:1077–1089, 1999.
Thatipelli, M. R., E. A. Sabater, H. Bjarnason, M. A. McKusick, and S. Misra. CT angiography of renal artery anatomy for evaluating embolic protection devices. J. Vasc. Interv. Radiol. 18:842–846, 2007.
Thomas, J. B., L. Antiga, S. L. Che, J. S. Milner, D. A. Steinman, J. D. Spence, and B. K. Rutt. Variation in the carotid bifurcation geometry of young versus older adults: implications for geometric risk of atherosclerosis. Stroke 36:2450–2456, 2005.
Thomas, J. B., J. S. Milner, and D. A. Steinman. On the influence of vessel planarity on local hemodynamics at the human carotid bifurcation. Biorheology 39:443–448, 2002.
Timmins, L. H., C. A. Meyer, M. R. Moreno, and J. E. Moore, Jr. Mechanical modeling of stents deployed in tapered arteries. Ann. Biomed. Eng. 36:2042–2050, 2008.
Verschuyl, E. J., R. Kaatee, F. J. Beek, G. Pasterkamp, W. H. Bush, J. J. Beutler, P. J. van der Ven, and W. P. Mali. Renal artery origins: location and distribution in the transverse plane at CT. Radiology 203:71–75, 1997.
Wake, A. K., J. N. Oshinski, A. R. Tannenbaum, and D. P. Giddens. Choice of in vivo versus idealized velocity boundary conditions influences physiologically relevant flow patterns in a subject-specific simulation of flow in the human carotid bifurcation. J. Biomech. Eng. 131:021013, 2009.
Weld, K. J., S. B. Bhayani, J. Belani, C. D. Ames, G. Hruby, and J. Landman. Extrarenal vascular anatomy of kidney: assessment of variations and their relevance to partial nephrectomy. Urology 66:985–989, 2005.
Wijesinghe, L. D., D. J. Scott, and D. Kessel. Analysis of renal artery geometry may assist in the design of new stents for endovascular aortic aneurysm repair. Br. J. Surg. 84:797–799, 1997.
Wood, N. B., S. Z. Zhao, A. Zambanini, M. Jackson, W. Gedroyc, S. A. Thom, A. D. Hughes, and X. Y. Xu. Curvature and tortuosity of the superficial femoral artery: a possible risk factor for peripheral arterial disease. J. Appl. Physiol. 101:1412–1418, 2006.
Yahel, J., and B. Arensburg. The topographic relationships of the unpaired visceral branches of the aorta. Clin. Anat. 11:304–309, 1998.
Yim, P. J., J. R. Cebral, A. Weaver, R. J. Lutz, O. Soto, G. B. Vasbinder, V. B. Ho, and P. L. Choyke. Estimation of the differential pressure at renal artery stenoses. Magn. Reson. Med. 51:969–977, 2004.
Zhu, H., Z. Ding, R. N. Piana, T. R. Gehrig, and M. H. Friedman. Cataloguing the geometry of the human coronary arteries: a potential tool for predicting risk of coronary artery disease. Int. J. Cardiol. 135:43–52, 2009.
Acknowledgments
The authors would like to acknowledge Ms. Geraldine Dowd, Clinical Specialist Radiographer, University College Hospital, Galway for her help, and James Coburn for his technical expertise. This study was supported with funds from Irish Research Council for Science, Engineering, and Technology (IRCSET): funded by the National Development Plan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael B. Lawrence oversaw the review of this article.
Rights and permissions
About this article
Cite this article
O’Flynn, P.M., O’Sullivan, G. & Pandit, A.S. Geometric Variability of the Abdominal Aorta and Its Major Peripheral Branches. Ann Biomed Eng 38, 824–840 (2010). https://doi.org/10.1007/s10439-010-9925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-010-9925-5