Abstract
More than half of the European population of the Barbary Partridge is in Sardinia; nonetheless, the researches concerning this species are very scarce, and its conservation status is not defined because of a deficiency of data. This research aimed to analyse the habitat selection and the factors affecting the abundance and the density of the Barbary Partridge in Sardinia. We used the data collected over 8 years (between 2004 and 2013) by spring call counts in 67 study sites spread on the whole island. We used GLMM to define the relationships between the environment (topography, land use, climate) both the occurrence and the abundance of the species. Moreover, we estimated population densities by distance sampling. The Barbary Partridge occurred in areas at low altitude with garrigue and pastures, avoiding woodlands and sparsely vegetated areas. We found a strong relationship between the occurrence probability and the climate, in particular, a positive relation with temperature and a negative effect of precipitation, especially in April–May, during brood rearing. Furthermore, dry crops positively affected the abundance of the species. We estimated a density of 14.1 partridges per km2, similar to other known estimates. Our findings are important both because they increase the knowledge concerning this species, which is considered data deficient in Italy, and because they are useful to plan management actions aimed to maintain viable populations if necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Barbary Partridge (Alectoris barbara Reichenow 1896) is one of the four species of the genus Alectoris found in the Mediterranean Basin. It is distributed in northwestern Africa, from Western Sahara east to Libya, as well as at Gibraltar, in the Canary Islands and in Sardinia, where it was probably introduced historically (Cramp 1980; Scandura et al. 2010). Generally, the species inhabits dry and open lands with scrubs, rocky areas, coastal dunes, shrubs and maquis, but it is also present in open woodlands, pine forests and croplands (Cramp 1980; del Hoyo et al. 1994; Keller et al. 2020). The European population is estimated at 7500–20,000 pairs, more than half present in Sardinia (Italy) with 5000–10,000 breeding pairs and a decreasing population trend (BirdLife International 2017). For this reason, the species was classified as SPEC 3 (BirdLife International 2017). The main threats for its conservation are the over-hunting and habitat loss caused by agricultural intensification, habitat encroachment caused in turn by the decrease of grazing pressure and urbanization (Brichetti and Fracasso 2004; del Hoyo et al. 2013). However, in Italy, the conservation status of the species is not evaluated by IUCN (International Union for Conservation of the Nature) because it is ‘data deficient’ (DD) (Rondinini et al. 2013); therefore, any new study concerning this species is worthy.
For this reason, we aimed to investigate the environmental factors affecting the distribution and the density of the species during the breeding season in Sardinia. In particular, by using data collected between 2004 and 2013 in 67 study sites, we (i) analyzed the habitat selection of the species, (ii) investigated the relationships between abundances and the environment and (iii) estimated the breeding densities. This research is relevant for many reasons. First, despite Sardinia holds more than half of the European population (58%; BirdLife International 2017), there are few studies about the ecology of this species in Sardinia, most of local interest (Castaldi and Guerrieri 1997; Guerrieri 1997; Murgia and Murgia 2003; Luchetti et al. 2005; Chiatante et al. 2020). Moreover, little is known about this species even in other contexts (Akil and Boudedja 2001; Hanane 2020). Second, as well as exploring the importance of land use on the species distribution, we also tested the effect of climate variables, which are very important because climate strongly affect Galliform populations (Potts 2012) and the research concerning this topic, and the related climate change is only in an early stage (Tian et al. 2018). Third, this study presents the largest survey ever carried out on the species in Europe, including almost all the environments and climatic contexts of Sardinia. Finally, being the Barbary Partridge a gamebird species, it is essential to know the environmental factors affecting its distribution and density in order to define sustainable hunting pressure and to plan management actions aimed to maintain viable populations (Sinclair et al. 2006; Sands et al. 2012).
Materials and methods
Study area
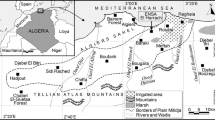
This study was carried out in Sardinia (Mediterranean Sea, Italy) (39° 58′ N, 9° 01′ E) (Fig. 1), which extends for 24,100 km2. The altitude ranges from sea level to 1834 m a.s.l. (Punta La Marmora, in the Gennargentu massif), and the climate is Mediterranean, with a mean yearly temperature of 18 °C (min. 11 °C in December, max. 26 °C in August) and mean yearly precipitation ranging from 20 mm in July to 210 mm in December (Chessa and Delitala 1997). Vegetation is typically Mediterranean: the landscape is dominated by woodlands (20.9%), mainly of holm oak (Quercus ilex) and cork oak (Quercus suber), garrigue and Mediterranean maquis (23.3%) (composed of Arbustus unedo, Phillyrea sp., Pistacia lentiscus, Cistus spp., Erica arborea, Myrtus communis) and natural grasslands (6.0%). Agricultural areas are composed mainly of arable lands (dry crops, horticulture) and meadows, representing 19.2% and 7.3% respectively, followed by heterogeneous agricultural areas (8.3%) and permanent crops (3.5%); urban areas covered only 3.0% of the island.
Data collection was carried out in 67 study sites (average size ± SD 12.0 km2 ± 9.15; min. = 2.3, max. = 50.1 km2), placed in the whole Sardinia at an average distance of 7.6 km each other (SD = 7.7, min. = 1.2 km, max. = 44.5 km), and covering a total surface of 802 km2. Their landscape is representative of the island; indeed, they are mainly composed of Mediterranean maquis (16.9%), garrigue (13.2%), woodlands (15.6%) and not irrigated arable lands (14.3%) (Fig. 1, Table 1). Most of them (N = 44) were protected areas in which hunting was forbidden while the other ones (N = 23) were hunting districts in which partridge shooting was permitted during a very limited hunting season (2 days per year). In hunting districts, the cover of heterogeneous agricultural areas and woodlands was higher than protected areas (Electronic Supplementary Materials, ESM Table S1).
Fieldwork
Data concerning the distribution and the breeding density of the species were collected in springs 2004–2013, excluding 2008 and 2011, by call counts from listening points (Bibby et al. 2000; Sutherland et al. 2004), conducted once between late March and early May. During these 8 years, we carried out 1248 listening points, which were placed following a simple random sampling design, in proportional number with respect to the study site surface (Krebs 1999; Barabesi and Fattorini 2013) (ESM Table S1). The number of points in the same site but in different years changed due to the number of available observers; for the same reason, each site was sampled mainly for 2 years (range 1–4 years/site). Particularly, 59 expert observers were employed during the 8-year fieldwork, with a minimum of 11 (in 2011) and a maximum of 33 (in 2004) per year, with 22 of them (37.3%) employed more than 1 year; they carried out the listening points without a planned scheduling, having in this way multiple observers per site in the same year (Alldredge et al. 2006). We placed the listening points at a minimum distance of at least 300 m (average distance within any given years, min = 512 m, max = 841 m) to avoid spatial correlation and double counts (Blondel et al. 1981; Ralph et al. 1995; Sutherland et al. 2004). Call counts were carried out during the first three hours after dawn (6:00 a.m.–9:00 a.m.) and the last two hours before dusk (6:30 p.m.–8:30 p.m.). We used the playback to increase the detectability of males (Bibby et al. 2000; Jacob et al. 2010; Chiatante et al. 2013) amplified with a portable game caller (Multisound D8 Pocket; Multisound S.n.C., Italy). Listening and playback times were subdivided as follows: 3-min listening, 1-min playback, 1-min listening, 1-min playback, 1-min listening, 1-min playback and 2-min listening (Chiatante et al. 2020). We recorded all calling partridges, their exact or approximate location and the exact time of each call. When we observed single partridges or pairs, we measured the exact distance from the observer to the birds with a laser rangefinder (Leica Rangemaster 900; Leica, Germany). When we did not see a calling bird, we mapped the approximate position of the calling bird on aerial photographs (1:5000 scale) based on the likely attenuation and direction of its vocalization. We then measured the distance from the observer to the position of each calling bird using the software QGIS v.3.8.3 ‘Zanzibar’.
Environmental variables
We analysed the spatial distribution of the Barbary Partridge using variables related to topography, land use cover and climate (Table 1). In particular, we measured the environmental variables in a 300-m circular plot around each sampling points, as this size was used in previous researches concerning this species (Guerrieri 1997; Chiatante et al. 2020). The digital elevation model (DEM) of Sardinia, with a spatial resolution of 10 m (available at http://www.sardegnageoportale.it/), was used to measure the topographic variables. Specifically, we calculated the mean altitude, the mean slope and the dominant exposition (i.e. the exposition occurring in the majority of 10-m pixels contained in each 300-m plot). Thirteen land use variables were measured as percentage cover by the land use cover map of Sardinia Region (scale 1:25,000, year 2008), which was built with orthophotos collected between 2003 and 2006. Considering that the data of partridges were collected until 2013, the validation of the land cover map was based on the visual interpretation of high-resolution images on Google Earth (© Google LLC; available at https://www.google.com/earth/index.html) (Dorais and Cardille 2011; Zhao et al. 2014), highlighting no evident changes over the years. Besides, the habitat heterogeneity was calculated by Shannon index of diversity (Magurran 2004) using a spreadsheet of Microsoft Excel 2016 (Microsoft Corporation 2016). We did not explore the effect of other landscape metrics because previous research found no significant effects of this group of variables (Chiatante et al. 2020). Climate variables were derived by WorldClim 2.1 (Fick and Hijmans 2017), a dataset of spatially interpolated monthly climate data representative of the current climate for global land areas at a very high spatial resolution (approximately 1 km2). In particular, we used four bioclimatic variables: the annual mean temperature (BIO01), the temperature seasonality (i.e. standard deviation of mean temperature × 100; BIO04), the annual precipitation (BIO12) and the precipitation seasonality (i.e. coefficient of variation of precipitation; BIO15). Moreover, from the same dataset, we used the solar radiation and both the temperatures and precipitation of April and May, which were averaged obtaining the temperature of April–May and precipitation of April–May. In fact, likewise other Galliforms, partridges are affected negatively by weather conditions during the breeding season (Pleasant et al. 2006; Potts 2012; Guttery et al. 2013) and April–May is the main period of incubation and hatching for this species in Italy (Brichetti and Fracasso 2004). We tested also the importance of the protection level of the site, i.e. protected areas vs hunting districts. All the environmental variables were measured by QGIS v.3.8.3 Zanzibar and R v.3.6.1 (R Core Team 2019), and the related packages sp (Pebesma and Bivand 2011) and raster (Hijmans et al. 2014).
Data analyses
Barbary Partridge occurrence
We used generalized linear mixed models (GLMMs) (Zuur et al. 2009; Harrison et al. 2018) with a binary distribution (link function = logit) to relate the presence of the Barbary Partridge with the environmental variables. The presence of the species was 1 when at least one individual was detected in the 300-m plot around the listening points, or 0 otherwise. We built random intercept models using as random effects the sites (within which listening points were nested) crossed per years to control for the non-independence of data, through a partially crossed design (Schielzeth and Nakagawa 2013). Barr et al. (2013) suggest fitting the maximal random effect structure possible (the ‘keep it maximal’ rule), even if Harrison et al. (2018) suggest fitting the most complex structure allowed by your data. For this reason, we did not build random slope models because they require large numbers of data and are unstable when, as in our case, sample sizes across groups are unbalanced (Harrison et al. 2018). However, to build the most complex and parsimonious model, we proceeded in two steps to choose the fixed effect structure. First, we selected only the variables with a remarkable effect on the partridge occurrence (therefore with some evidence of importance), with a pairwise comparison of the second-order Akaike information criterion (AICc; Akaike 1973) of two simple GLMMs: one with the intercept only and the other with each variable (Burnham et al. 2011). When the AICc value of the GLMM with the variable was lesser than the one with the intercept only, with a difference of at least two (Δ AICc ≤ 2), that variable was retained (Burnham and Anderson 2002). Once the number of variables was reduced, we ran a priori sets of GLMM built with all combinations of the retained environmental variables; these models were built using not correlated variables (|r|< 0.70). Then, for each model, the AICc was calculated and the models with the lowest AICc were selected as the best (Burnham and Anderson 2002; Grueber et al. 2011). Specifically, we considered as the best, models included within the ‘95% confidence set’ based on cumulative Akaike weights (Burnham and Anderson 2002; Harrison et al. 2018). These models were averaged, and the importance of each variable in the set was calculated (Burnham and Anderson 2002; Harrison et al. 2018). For this analysis, all the variables considered were standardized by normalization; that is, each variable had a mean of zero and a standard deviation of one (Quinn and Keough 2002; Zuur et al. 2007). We used the variance inflation factor (VIF) with a threshold of three to exclude the collinearity among variables (Fox and Monette 1992). We calculated the average of the Pearson’s residuals (Harrison et al. 2018) of each model in the best subset and tested them for spatial autocorrelation by the Moran I test (Zuur et al. 2007; Bivand et al. 2008). The coefficient of determination R2 was used as a measure of the variance explained: in particular, we calculated the marginal R2, for the variance explained by only the fixed effects, and the conditional R2, which encompasses the variance explained by both fixed and random effects (Nakagawa and Schielzeth 2013; Nakagawa et al. 2017). The discrimination ability of the average model was assessed through the area under the curve (AUC) of the ROC (receiver operating characteristic) plot (Pearce and Ferrier 2000; Fawcett 2006). Finally, we predicted the probability of occurrence of the Barbary Partridge in Sardinia. Precisely, using a grid covering the whole island and with a spatial resolution of 532 m (i.e. comparable to the surface of the 300 m plots), first we measured for each square of the grid the environmental variables included in the model. Then, after their standardization (as we did to build the models), we reclassified these values using the coefficients estimated by the average model, but without considering the effect of the random variables. In this way, we obtained the probability of occurrence of the species for each square of the grid, allowing us to calculate the average (± SD) probability of occurrence of the Barbary Partridge in the whole island. All analyses were performed using the statistical software R v.3.6.1 (R Core Team 2019) and the packages lme4 (Bates et al. 2015), MuMIn (Bartoń 2018), car (Fox and Weisberg 2011), spdep (Bivand et al. 2015) and verification (NCAR - Research Applications Laboratory 2015).
Barbary Partridge abundance
We used GLMMs (Zuur et al. 2009; Harrison et al. 2018) with a negative binomial error distribution (link function = logarithmic) to relate the abundance of the Barbary Partridge with the environmental variables. The abundance of the species was calculated as the number of partridges observed in the 300-m plot around the sampling points in each count (i.e. one count for each point, for each year). We built the most complex and parsimonious model as we did for the occurrence model (see the previous paragraph “Barbary Partridge occurrence”). Therefore, first, we reduced the number of variables by a pairwise comparison; then, we ran a set of GLMMs built with all combinations of the retained and not correlated (|r|< 0.70) environmental variables, and averaged the best ones included in the ‘95% confidence set’ based on cumulative Akaike’s weights. We calculated the VIF to verify the absence of collinearity and tested the spatial correlation of Pearson’s residuals by Moran I test. The coefficient of determination R2 was used as a measure of the variance explained, and the goodness of fit was calculated through Pearson’s correlation between observed and predicted values (Rodríguez-Caro et al. 2016; Ondei et al. 2020; Chiatante and Panuccio 2021). Finally, as calculated for the probability of occurrence, using the coefficients of the average model, but without considering the effect of the random variables, we predicted the abundance of the Barbary Partridge in a grid covering the Sardinia with spatial resolution of 532 m; then, we calculated its average value for the whole island. All analyses were performed using the statistical software R v.3.6.1 (R Core Team 2019) and the packages listed in the previous paragraph.
Barbary Partridge density
The density of the species was estimated through the distance sampling method, particularly the conventional distance sampling (CDS) (Buckland et al. 1993). After a visual inspection of distances distribution, we truncated the 10% of the greatest distances as suggested by Buckland et al. (1993) and transformed the distance data into equal intervals of 60 m. As suggested by Buckland et al. (1993) and Thomas et al. (2010), we tested the following combinations of key functions and series adjustments: (1) uniform key with cosine adjustments, (2) half-normal key with cosine adjustments, (3) half-normal key with Hermite polynomial adjustments and (4) hazard-rate key with simple polynomial adjustments. Anyway, the probability of detecting a bird depends not only on distance but also on many other factors, such as habitat, weather, observer and bird behaviour (Buckland et al. 1993), a circumstance that could exist, at least in part, in this research due to the spatio-temporal variability of our data. Therefore, ignoring all these other factors, besides distances, could cause some bias in the estimate (Beavers and Ramsey 1998; Bas et al. 2008; Anderson et al. 2015). For this reason, besides CDS, we used also multiple covariate distance sampling (MCDS) (Marques et al. 2007), an extension of CDS that allow modelling the detection probability as a function of variables other than distance. Therefore, a graphical exploratory analysis was run to assess if the habitat around points and the year might biased the estimate of the density. For the habitat, we considered the cover of open areas (not-irrigated arable lands, meadows, horticulture, pastures and sparsely vegetated areas), scrublands (Mediterranean maquis, garrigue, shrublands) and woodlands in the 300-m circular plot around the sampling points (for more detail, see the paragraph “Environmental variables”). The results of this analysis (ESM Fig. S1) showed that both years (in particular 2012) and the cover of woodlands could bias our estimate because the detection distance changed with them. However, the year is a factor covariate with eight levels, too many for assessing the goodness of fit of the model with the bins obtained by grouping data (see further details below) (Marques et al. 2007); therefore, we did not use the year as a covariate but discarded data collected in 2012 (in which detection distances were lower than other years) to overcome this problem. Consequently, we used as covariate only the cover of woodland. To estimate density with MCDS, only half-normal and hazard rate keys are allowed (Marques et al. 2007). We built the detection functions using the protection levels as strata, i.e. protected areas vs hunting districts; we did not use sites as strata because for many of them there were too few observations and estimates would have not been reliable. For this reason, we preferred to calculate an accurate global detection probability, which was then used to calculate manually the density (see further details below), rather than estimate a global geographically biased density. Once the models were computed, ran both with CDS and MCDS, we used Akaike’s information criterion (AIC) to select the best model (Buckland et al. 1993; Thomas et al. 2010). Considering that data were binned, the goodness of fit of the models was assessed by χ2 tests (Buckland et al. 1993; Thomas et al. 2010). Furthermore, the average probability of detection was estimated and the effective detection radius (EDR) was defined. Finally, we calculated manually the density for each study site by dividing the number of observations across all points and years at the considered site by the surface of the covered area (i.e. the number of points sampled at the site multiplied EDR estimated). Similarly, we estimated density between protected areas and hunting districts. In this way, we obtained more accurate estimation of density, reflecting the geographic variation across all the Sardinia and the protection level of the area. The analyses were performed using the statistical software R v.3.6.1 (R Core Team 2019) and the package Distance (Miller et al. 2019).
Results
Barbary Partridge occurrence
The Barbary Partridge occurred in 54 study sites (80.6%) and in 527 points (42.2%) (Fig. 1). The pairwise comparison suggested that 12 environmental variables affected in a remarkable way the species occurrence (ESM Tables S2-S3, Figs. S2-S3). These encompassed both topography, land use and all the climatic variables considered. Indeed, we found the species in areas at low altitude, with garrigue and pastures, especially in warmer and dryer sites with reduced seasonality (ESM Figs. S2-S3). The land use diversity and the protection of the site did not affect the species occurrence. The average GLMM showed that six variables affected in a marked way the Barbary Partridge occurrence (Table 2, Fig. 2, ESM Fig. S4, ESM Table S5). In particular, garrigues and pastures increased its probability of occurrence while olive groves, woodlands and sparsely vegetated areas decreased it. Moreover, the temperature in April–May had a positive effect on the species occurrence. The VIF revealed no collinearity among variables (Table 2), and the model residuals did not show any spatial correlation (Moran test, I = 0.191, P = 0.424). The conditional R2 explained on average 51.2% of the variance, whereas the marginal R2 explained on average 14.5%. ROC analysis showed a good ability of the model to distinguish between occupied and not occupied points (AUC = 0.887, P < 0.001). The average probability of occurrence estimated in Sardinia by the model was equal to 0.385 ± 0.198 (SD) (min. 0.006, max. 0.864), with higher values in lowlands (ESM Fig. S5). The probability of occurrence was similar between protected areas (median ± IQR; 0.39 ± 0.59) and hunting districts (0.39 ± 0.45) (Mann–Whitney U test, P = 0.087).
Barbary Partridge abundance
Between 2004 and 2013, we collected 1459 observations of 1582 partridges. The pairwise comparison showed that 10 environmental variables affected in a remarkable way the species abundance (ESM Table S2, Figs. S6-S7). These encompassed both topography, land use and all the climate variables used. Indeed, we found higher abundances of the species in areas at low altitude, with not irrigated arable lands and without woodlands. Besides, the Barbary Partridge abundance was positively related to warmer and dryer areas with good solar radiation (ESM Figs. S6-S7). The land use diversity and the protection of the site did not affect the species abundance. The average GLMM showed that five variables affected the abundance of the species (Table 3, Fig. 3, ESM Fig. S8, ESM Table S6). In particular, Barbary Partridge was more abundant in not irrigated arable lands, avoiding woodlands. Besides, the temperature in April–May and solar radiation were positively related to its abundance, which was affected negatively by temperature seasonality. The VIF revealed no collinearity among variables (Table 3) and the model residuals did not show any spatial correlation (Moran test, I = 0.394, P = 0.347). The conditional R2 explained on average 18.5% of the variance, whereas the marginal R2 explained on average 7.5%. The goodness of fit of the model was good; indeed, the observed and the predicted abundances were positively correlated (r = 0.542, P < 0.001). The average number of partridges estimated in Sardinia by the model was equal to 2.4 per 300-m buffer, equal to a density of 8.6 ind./km2 ± 0.537 (SD) (min. 3.9, max. 18.6) and, following the occurrence model, showed higher values in lowlands (ESM Fig. S9). The estimated abundance was almost the same between protected areas (median ± IQR; 2.4 ± 1.00) and hunting districts (2.4 ± 1.13) (Mann–Whitney U test, P = 0.800).
Density of the Barbary Partridge
We collected 1200 observations of 1323 partridges, excluding 2012 (see the previous paragraph “Barbary Partridge density”). On average, 1.1 partridges per point were detected (SE = 0.013, min = 1, max = 6). The best detection probability function was the half-normal cosine without woodland cover as covariate (Table 4; Fig. 4) (χ2 = 0.291, df = 2, P = 0.865); this gave an EDR equal to 152 m. Using this radius, the surface reference was equal to 0.07 km2; therefore, the global average density estimated was 14.1 ind./km2 (SE = 1.88, min = 0, max = 71.0) (for estimates of each site, see ESM Table S7). The estimated density in protected areas was 16.2 ind./km2 (SE = 2.38, min = 0, max = 71.0), whereas in hunting districts was 15.0 ind./km2 (SE = 3.18, min = 0, max = 52.4).
Discussion
This research was aimed to explore how the environment (topography, land use and climate) affects the occurrence and abundances of Barbary Partridges in Sardinia and to estimate its breeding density. The study was based on data collected for 8 years in 67 sites distributed throughout the island. The study highlighted the importance of some environmental characteristics in influencing both the probability of presence and the abundance of the species. We found that the species selects areas with garrigue and pastures, and with low cover of woodlands, olive groves and sparsely vegetated areas (i.e. bare rocks, cliffs and beaches). This is not surprising inasmuch it is known that the species lives mainly in open areas, such as pastures, with shrubs and garrigue, even though a low cover of woodland is tolerated (Cramp 1980; Brichetti et al. 1992; Brichetti and Fracasso 2004; Hanane 2019). Moreover, univariate analysis showed that the species occurs with higher abundances at low altitude, as found both in north-eastern Sardinia (Chiatante et al. 2020) and in southern Sardinia, where the species had higher densities within 250 m a.s.l. (Murgia and Murgia 2003). Generally, the altitude seems not very important for the species, also occurring up to 3300 m in High Atlas (Cramp 1980), despite in the last century in Sardinia, it was observed a tendency to shift its range from lowlands towards hills, up to 600–700 m (Brichetti et al. 1992). As previously said, the species lives in open areas with shrubs, as has been confirmed by Chiatante et al. (2020) in north-eastern Sardinia, a pattern found also in the present research; indeed, the Barbary Partridge occurrence is positively related to garrigue and pastures. The garrigue is likely to be used as a refuge from predators and to conceal the nest, whereas pastures are necessary to forage and to guarantee a good source of arthropods for chicks (Potts 2012). This is common in the Mediterranean Basin also for a similar Alectoris species (e.g. red-legged partridge Alectoris rufa; Chiatante et al. 2013). The positive relation between partridges and shrub cover was also found in North Africa, being the main factor driving the nest site selection and the breeding performance of the Barbary Partridge (Hanane 2019, 2020). Generally, the species occurs also in croplands and, in Sardinia, especially in the lowland (Brichetti et al. 1992; Hanane 2020). Our results highlight the importance of not irrigated arable lands for the species abundance, which were mainly composed of cereal crops. This fact could suggest that not irrigated arable lands are supplementary habitat for the species (sensu Dunning et al. 1992), at least in Sardinia. In fact, the species can persist also in areas without croplands, but in association with garrigue and pastures, which provide an additional amount of suitable habitat allowing partridge populations to reach higher abundances. However, it is reasonable to think that these habitats could be considered suboptimal and sink areas in which the breeding success is quite low (Hammerquist Wilson and Crawford 1987; Aldridge and Boyce 2007).
Regarding the climate, as expected, both univariate and multivariate analyses showed that it affects both the species occurrence and abundance. In general, the species inhabits areas with high temperatures and low precipitations during the whole years. This is not surprising, in fact, the Barbary Partridge is well adapted to live in warm and dry conditions (Huntley et al. 2007). The selection for dryer conditions is underlined also by the positive effect of the solar radiation for the species abundance that we found with univariate analyses, which confirms what Chiatante et al. (2020) supposed in the north-eastern Sardinia. Indeed, in this area, the species selects slopes facing to the east that receive higher solar radiation than other exposures, resulting in higher evapotranspiration rates and higher temperature (Kirkby et al. 1990; Adams 2007; Zahran and Gilbert 2010). Another important result is the strong positive effect of temperature and, especially, the negative effect of precipitation during April–May, which is the main period of incubations and hatchings of this species in Italy (Brichetti and Fracasso 2004). Generally speaking, it is known that the short-term annual variations in weather affect the population dynamic of birds (Dempster 1975; Elkins 2004). As a matter of fact, the weather in spring and summer influences the bird numbers over the next seasons (Golovatin 2002; Enemar et al. 2004). Obviously, these negative effects of weather on birds are more evident in extreme landscapes (Greenwood and Baillie 1991; Robinson et al. 2014; Walther et al. 2017). Likewise other birds, also partridges are negatively affected by bad weather conditions both on breeding densities (Potts 2012) and on the time spent in the vocal activity, as found for the red-legged partridge, the grey partridge (Perdix perdix) and the northern bobwhite (Colinus virginianus) (Green 1984; Pépin and Fouquet 1992; Wellendorf et al. 2004). Similarly, bad weather is a limiting factor that reduces the lek attendance of the greater sage grouse (Centrocercus urophasianus) and the sharp-tailed grouse (Tympanuchus phasianellus) (Bradbury et al. 1989; Drummer et al. 2011; Fremgen et al. 2019). In addition, the breeding success is low with bad weather conditions (Erikstad and Spidso 1982; Montagna and Meriggi 1991; Zheng-Ji and Yue-Hua 1997; Pleasant et al. 2006; Guttery et al. 2013). Nonetheless, many researches showed that the rainfalls prior to hatching enhance chick survival, perhaps because wet weather increases the nest concealment and the arthropod fauna availability (Erikstad and Andersen 1983; Lucio 1990; Goddard and Dawson 2009; Caudill et al. 2014).
It is important to underline the absence of relation with the protection level of the area both for occurrence and abundance, as found also by previous research (Chiatante et al. 2020). However, in protected areas, there was a slightly higher density than hunting districts (16.2 vs 15.0 ind./km2). This weak difference is likely related to the quite similar environmental characteristics of sites and the very low hunting pressure on the species (2 days for hunting season). However, it is known that, even in these circumstances, the conservation of a game species could be threatened (De Leo et al. 2004), also considering that in Sardinia, poaching is a serious threat (Brochet et al. 2016) and that regulatory measures exist but are enforced due to an insufficient number of wardens (BirdLife International 2016).
For the period 2004–2013, we estimated a density equal to 14.1 ind./km2 for the Barbary Partridge (hence, approximately 7.0 pairs/km2), an estimate quite high respect to other studies (0.6–1.5 pairs/km2, Murgia and Murgia 2003) and in line with others (8.3–14.3 pairs/km2, Luchetti et al. 2005; 5.1–22.9 pairs/km2, Chiatante et al. 2020). Similar densities (8.3 pairs/km2) were estimated also in Algeria (Akil and Boudedja 2001). However, these differences arise likely because previous researches in Sardinia were carried out in small areas possibly not representative of the whole island. We think that our estimate is quite plausible, because of the large sample size and because we analysed data following all suggestions available in the literature to avoid biases (Prieto Gonzalez et al. 2017). Anyway, despite the population trend in Italy is declining (Nardelli et al. 2015), our results showed an estimate in line with the threshold value of 6–7 pairs/km2 which should be used to evaluate as ‘Favourable’ the status of the species population at a local scale (Gustin et al. 2016). Finally, we argued about the different estimates obtained with 300-m buffers (8.6 ind./km2) and distance sampling (14.1 ind./km2). Many studies have found differences between indices of relative abundances calculated within fixed-radius plots (such as the buffer of 300 m) and distance sampling estimates (Norvell et al. 2003; Buckland et al. 2008; Gottschalk and Huettmann 2011). All of them stressed the more convincing estimates of distance sampling, which gives precise unbiased results respect to a study design based on a relative abundance approach, in as much it takes into account the detectability of individuals (Buckland et al. 1993). However, relative abundances reflect species responses to ecological gradients and can be easily employed to investigate trends, therefore are very useful in some circumstances (Hutto and Young 2003; Johnson 2008; Banks-Leite et al. 2014).
In conclusion, the Barbary Partridge in Sardinia occurs in areas with garrigue and pastures, especially at low altitude, with densities of 14.1 ind./km2 (slightly higher in protected areas than hunting districts), and with higher abundances where dry arable lands are available. These findings are important in the view of the species conservation status. First, could improve the knowledge required to define its status assessment. Indeed, as previously said, the species is classified by IUCN as data deficient (Rondinini et al. 2013) and there is a lack of information to establish its Favourable Reference Value for breeding populations at a regional scale in Italy (Gustin et al. 2016). Then, knowing its ecology, distribution and status may help conservation biologists to plan management actions aimed to maintain viable populations if necessary, such as habitat improvement actions and restocking programs (BirdLife International 2016). For instance, our findings could be useful to plan habitat improvement actions like game crops, which can be created to support the species populations, particularly in areas where the abundances are low. Indeed, in Portugal, the similar red-legged partridges living in areas of agricultural abandonment are favoured by the presence of legume game crops (Reino et al. 2016). On the other hand, our models can be used to address in a sustainable way restocking programs of weak populations, for instance in areas with a high probability of species occurrence, as done for other Alectoris species (Meriggi et al. 2007). Moreover, we highlighted the importance of climate on the species occurrence and abundance, because the species is disadvantaged by low temperature and high precipitation, especially during the breeding season. Our findings are relevant also in view of climate changes. Indeed, the projections of the IPCC (Intergovernmental Panel on Climate Change) showed a trend toward the increase of temperature and the decrease of precipitation in the Mediterranean Basin and in Italy, even though also indicate general increases in the intensity and frequency of extreme precipitation (IPCC 2013; Desiato et al. 2015). In this context, the species could be favoured in the next decades, despite some studies argue that the species will be extinct in the next future in Europe (Huntley et al. 2007).
Data availability
Data collection did not involve sampling procedure, and experimental manipulation of birds and the fieldwork was conducted under the Law of the Republic of Italy on the Protection of Wildlife (February 25, 1992).
References
Adams JM (2007) Vegetation-climate interaction: how vegetation makes the global environment. Springer, Berlin, New York
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second International Symposium on Information Theory. Akademiai Kiado, Budapest, pp 267–281
Akil M, Boudedja S (2001) Reproduction de la perdrix gambra (Alectoris barbara) dans la région de Yakouren (Algérie). Game Wildl Sci 18:459–467
Aldridge CL, Boyce MS (2007) Linking occurrence and fitness to persistence: habitat-based approach for endangered Greater Sage-grouse. Ecol Appl 17:508–526
Alldredge MW, Pollock KH, Simons TR (2006) Estimating detection probabilities from multiple-observer point counts. Auk 123:1172–1182. https://doi.org/10.1093/auk/123.4.1172
Anderson AS, Marques TA, Shoo LP, Williams SE (2015) Detectability in audio-visual surveys of tropical rainforest birds: the influence of species, weather and habitat characteristics. PLoS ONE 10:e0128464. https://doi.org/10.1371/journal.pone.0128464
Banks-Leite C, Pardini R, Boscolo D et al (2014) Assessing the utility of statistical adjustments for imperfect detection in tropical conservation science. J Appl Ecol 51:849–859. https://doi.org/10.1111/1365-2664.12272
Barabesi L, Fattorini L (2013) Random versus stratified location of transects or points in distance sampling: theoretical results and practical considerations. Environ Ecol Stat 20:215–236. https://doi.org/10.1007/s10651-012-0216-1
Barr DJ, Levy R, Scheepers C, Tily HJ (2013) Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68:255–278. https://doi.org/10.1016/j.jml.2012.11.001
Bartoń K (2018) Package MuMIn: multi-model inference. www.cran.r-project.org, Wien
Bas Y, Devictor V, Moussus J-P, Jiguet F (2008) Accounting for weather and time-of-day parameters when analysing count data from monitoring programs. Biodivers Conserv 17:3403–3416. https://doi.org/10.1007/s10531-008-9420-6
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft 67. https://doi.org/10.18637/jss.v067.i01
Beavers SC, Ramsey FL (1998) Detectability analysis in transect surveys. J Wildlife Manage 62:948–957
Bibby CJ, Burgess ND, Hill DA, Mustoe SH (2000) Bird census techniques, 2nd edn. Academic Press, London, UK
BirdLife International (2017) European birds of conservation concern: populations, trends and national responsabilities. BirdLife International, Cambridge, UK
BirdLife International (2016) Alectoris barbara: The IUCN Red List of Threatened Species 2016: e.T22678707A85855433. https://doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22678707A85855433.en. Accessed 15 Mar 2021
Bivand R, Altman M, Anselin L, et al (2015) Package spdep: spatial dependence—weighting schemes, statistics and models. www.cran.r-project.org, Wien
Bivand R, Pebesma EJ, Gómez-Rubio V (2008) Applied spatial data analysis with R. Springer, New York
Blondel J, Ferry C, Frochot B (1981) Point counts with unlimited distance. Stud Avian Biol 6:414–420
Bradbury JW, Vehrencamp SL, Gibson RM (1989) Dispersion of displaying male sage grouse: I. patterns of temporal variation. Behav Ecol Sociobiol 24:1–14. https://doi.org/10.1007/BF00300112
Brichetti P, De Franceschi P, Baccetti N (eds) (1992) Fauna d’Italia. Aves I, Gavidae-Phasianidae. Edizioni Calderini, Bologna
Brichetti P, Fracasso G (2004) Ornitologia Italiana. Vol 2 - Tetraonidae-Scolopacidae. Alberto Perdisa editore, Bologna, IT
Brochet A-L, Van Den Bossche W, Jbour S et al (2016) Preliminary assessment of the scope and scale of illegal killing and taking of birds in the Mediterranean. Bird Conserv Intern 26:1–28. https://doi.org/10.1017/S0959270915000416
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling: estimating abundance of biological populations. Chapman & Hall, London
Buckland ST, Marsden SJ, Green RE (2008) Estimating bird abundance: making methods work. Bird Conserv Intern 18:91–108. https://doi.org/10.1017/S0959270908000294
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/s00265-010-1029-6
Castaldi A, Guerrieri G (1997) Il gregarismo della Pernice sarda, Alectoris barbara, nella Sardegna nord-orientale. Avocetta 21:28
Caudill D, Guttery MR, Bibles B, et al (2014) Effects of climatic variation and reproductive trade-offs vary by measure of reproductive effort in greater sage-grouse. Ecosphere 5:art154. https://doi.org/10.1890/ES14-00124.1
Chessa PA, Delitala A (1997) Il clima della Sardegna. Chiarella, Sassari (IT)
Chiatante G, Meriggi A, Giustini D, Baldaccini NE (2013) Density and habitat requirements of red-legged partridge on Elba Island (Tuscan Archipelago, Italy). Ital J Zool 80:402–411. https://doi.org/10.1080/11250003.2013.806601
Chiatante G, Panuccio M (2021) Environmental factors affecting the wintering raptor community in Armenia. Southern Caucasus Community Ecol First View Online: https://doi.org/10.1007/s42974-021-00038-7
Chiatante G, Vidus Rosin A, Cinerari CE et al (2020) Habitat selection and density of the Barbary partridge in Sardinia. Mediterranean Sea Eur J Wildlife Res 66:22. https://doi.org/10.1007/s10344-020-1360-9
Cramp S (ed) (1980) Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic. Oxford University Press, Oxford, New York
De Leo GA, Focardi S, Gatto M, Cattadori IM (2004) The decline of the grey partridge in Europe: comparing demographies in traditional and modern agricultural landscapes. Ecol Model 177:313–335. https://doi.org/10.1016/j.ecolmodel.2003.11.017
del Hoyo J, Elliot A, Sargatal J (eds) (1994) Handbook of the birds of the world. Vol.2 New World Vultures to Guineafowl. Lynx Edicions, Barcelona
del Hoyo J, Elliot A, Sargatal J et al (eds) (2013) Handbook of the birds of the world alive. Lynx Edicions, Barcelona
Dempster JP (1975) Animal population ecology. Academic Press, London
Desiato F, Fioravanti G, Fraschetti P, et al (2015) Il clima futuro in Italia: analisi delle proiezioni dei modelli regionali. ISPRA, Rome (IT)
Dorais A, Cardille J (2011) Strategies for incorporating high-resolution Google Earth databases to guide and validate classifications: understanding deforestation in Borneo. Remote Sens 3:1157–1176. https://doi.org/10.3390/rs3061157
Drummer TD, Corace RG, Sjogren SJ (2011) Sharp-tailed grouse lek attendance and fidelity in upper Michigan. J Wildlife Manage 75:311–318. https://doi.org/10.1002/jwmg.42
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Elkins N (2004) Weather and bird behaviour, 3rd edn. T & AD Poyser, London
Enemar A, Sjöstrand B, Andersson G (2004) The 37-year dynamics of a subalpine passerine bird community, with special emphasis on the influence of environmental temperature and Epirrita autumnata cycles. Ornis Svecica 14:63–106
Erikstad KE, Andersen R (1983) The effect of weather on survival, growth rate and feeding time in different sized Willow Grouse broods. Ornis Scand 14:249. https://doi.org/10.2307/3676311
Erikstad KE, Spidso TK (1982) The influence of weather on food intake, insect prey selection and feeding behaviour in Willow Grouse chicks in northern Norway. Ornis Scand 13:176. https://doi.org/10.2307/3676295
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874. https://doi.org/10.1016/j.patrec.2005.10.010
Fick SE, Hijmans RJ (2017) WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315. https://doi.org/10.1002/joc.5086
Fox J, Monette G (1992) Generalized collinearity diagnostics. J Am Stat Assoc 87:178–183. https://doi.org/10.2307/2290467
Fox J, Weisberg S (2011) Package car: an R companion to applied regression. www.cran.r-project.org, Wien
Fremgen AL, Hansen CP, Rumble MA et al (2019) Weather conditions and date influence male Sage Grouse attendance rates at leks. Ibis 161:35–49. https://doi.org/10.1111/ibi.12598
Goddard AD, Dawson RD (2009) Factors influencing the survival of neonate Sharp-Tailed Grouse Tympanuchus phasianellus. Wildlife Biol 15:60–67. https://doi.org/10.2981/07-087
Golovatin MG (2002) Population dynamics of passerines in the sub-Arctic conditions. Avian Ecol Behav 8:23–34
Gottschalk TK, Huettmann F (2011) Comparison of distance sampling and territory mapping methods for birds in four different habitats. J Ornithol 152:421–429. https://doi.org/10.1007/s10336-010-0601-1
Green RE (1984) The feeding ecology and survival of partridge chicks (Alectoris rufa and Perdix perdix) on arable farmland in East Anglia. J Appl Ecol 21:817–830
Greenwood JJD, Baillie SR (1991) Effects of density-dependence and weather on population changes of English passerines using a non-experimental paradigm. Ibis 133:121–133. https://doi.org/10.1111/j.1474-919X.1991.tb07675.x
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: challenges and solutions. J Evol Biol 24:699–711. https://doi.org/10.1111/j.1420-9101.2010.02210.x
Guerrieri G (1997) Habitat primaverile-estivo della Pernice sarda, Alectoris barbara, nella Sardegna nord-orientale. Avocetta 21:38
Gustin M, Brambilla M, Celada C (2016) Stato di conservazione e valore di riferimento favorevole per le popolazioni di uccelli nidificanti in Italia. Riv Ital Ornitol 86:3. https://doi.org/10.4081/rio.2016.332
Guttery MR, Dahlgren DK, Messmer TA et al (2013) Effects of landscape-scale environmental variation on Greater Sage-Grouse chick survival. PLoS ONE 8:e65582. https://doi.org/10.1371/journal.pone.0065582
Hammerquist Wilson M, Crawford JA (1987) Habitat selection by Texas Bobwhites and Chestnut-bellied Scaled Quail in South Texas. J Wildlife Manage 51:575–582
Hanane S (2020) Factors affecting the reproductive performance of barbary partridges in cereal croplands of Northwestern Morocco: the role of timing of breeding and vegetation cover at fine-scale. Biologia 75:235–241. https://doi.org/10.2478/s11756-019-00290-3
Hanane S (2019) Local versus landscape-scale determinants of nest-site selection in the Barbary Partridge Alectoris barbara. Bird Study 65:495–504. https://doi.org/10.1080/00063657.2018.1559797
Harrison XA, Donaldson L, Correa-Cano ME et al (2018) A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 6:e4794. https://doi.org/10.7717/peerj.4794
Hijmans RJ, van Etten J, Mattiuzzi M, et al (2014) Package raster: geographic data analysis and modeling. www.cran.r-project.org, Wien
Huntley B, Green RE, Collingham YC, Willis SG (2007) A climatic atlas of European breeding birds. Lynx Edicions, Barcelona
Hutto RL, Young JS (2003) On the design of monitoring programs and the use of population indices: a reply to Ellingson and Lukacs. Wildlife Soc B 31:903–910
IPCC (2013) Climate Change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (UK), New York (US)
Jacob C, Ponce-Boutin F, Besnarde A, Eraud C (2010) On the efficiency of using song playback during call count surveys of Red-legged partridges (Alectoris rufa). Eur J Wildlife Res 56:907–913. https://doi.org/10.1007/s10344-010-0388-7
Johnson DH (2008) In defense of indices: the case of bird surveys. J Wildlife Manage 72:857–868. https://doi.org/10.2193/2007-294
Keller V, Herrando S, Voříšek P et al (2020) European Breeding Bird Atlas 2: distribution, abundance and change. European Bird Census Council & Lynx Edicions, Barcelona
Kirkby MJ, Atkinson K, Lockwood J (1990) Aspect, vegetation cover and erosion on a semi-arid hillslopes. In: Thornes JB (ed) Vegetation and erosion. Wiley, New York, pp 25–39
Krebs CJ (1999) Ecological methodology, 2nd ed. Benjamin/Cummings, Menlo Park
Luchetti S, Contu S, Sacchi O, et al (2005) Demography of two barbary partridge (Alectoris barbara) populations in Sardinia Island. 27th Congress of the International Union of Game Biologist, Hannover, pp 398–399
Lucio AJ (1990) Influencia de las condiciones climaticas en la productividad de la Perdiz Roja (Alectoris rufa). Ardeola 37:207–218
Magurran AE (2004) Measuring biological diversity. Blackwell Pub, Malden, USA
Marques TA, Thomas L, Fancy SG, Buckland ST (2007) Improving estimates of bird density using multiple-covariate distance sampling. Auk 124:1229–1243
Meriggi A, Stella RM, della, Brangi A, et al (2007) The reintroduction of grey and red-legged partridges (Perdix perdix and Alectoris rufa) in central Italy: a metapopulation approach. Ital J Zool 74:215–237. https://doi.org/10.1080/11250000701246484
Microsoft Corporation (2016) Microsoft Excel. https://office.microsoft.com/excel
Miller DL, Rexstad E, Thomas L, et al (2019) Distance sampling in R. J Stat Soft 89:1–28. https://doi.org/10.18637/jss.v089.i01
Montagna D, Meriggi A (1991) Population dynamics of grey partridge (Perdix perdix) in northern Italy. Boll Zool 58:151–155
Murgia C, Murgia A (2003) Censimento al canto della pernice sarda Alectoris barbara barbara nell’oasi WWF di Monte Arcosu (2001–2002). Alula 10:86–91
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R 2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:20170213. https://doi.org/10.1098/rsif.2017.0213
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nardelli R, Andreotti A, Bianchi E, et al (2015) Rapporto sull’applicazione della Direttiva 147/2009/CE in Italia: dimensione, distribuzione e trend delle popolazioni di uccelli (2008–2012). ISPRA, Roma, IT
NCAR - Research Applications Laboratory (2015) Package verification: weather forecast verification utilities. www.cran.r-project.org, Wien
Norvell RE, Howe FP, Parrish JR (2003) A seven-year comparison of relative-abundance and distance-sampling methods. Auk 120:1013–1028. https://doi.org/10.1093/auk/120.4.1013
Ondei S, Prior LD, McGregor HW et al (2020) Small mammal diversity is higher in infrequently compared with frequently burnt rainforest–savanna mosaics in the north Kimberley. Australia Wildl Res First View Online: https://doi.org/10.1071/WR20010
Pearce J, Ferrier S (2000) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model 133:225–245. https://doi.org/10.1016/S0304-3800(00)00322-7
Pebesma E, Bivand R (2011) Package sp: classes and methods for spatial data. www.cran.r-project.org, Wien
Pépin D, Fouquet M (1992) Factors affecting the incidence of dawn calling in red-legged and grey partridges. Behav Process 26:167–176
Pleasant GD, Dabbert CB, Mitchell RB (2006) Nesting ecology and survival of Scaled Quail in the southern High Plains of Texas. J Wildlife Manage 70:632–640. https://doi.org/10.2193/0022-541X(2006)70[632:NEASOS]2.0.CO;2
Potts GR (2012) Partridges. Collins, London
Prieto Gonzalez R, Thomas L, Marques TA (2017) Estimation bias under model selection for distance sampling detection functions. Environ Ecol Stat 24:399–414. https://doi.org/10.1007/s10651-017-0376-0
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Wien
Ralph CJ, Sauer JR, Droege S (eds) (1995) Monitoring bird populations by point counts. Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture, Albany, CA, USA
Reino L, Borralho R, Arroyo B (2016) Influence of game crops on the distribution and productivity of red-legged partridges Alectoris rufa in Mediterranean woodlands. Eur J Wildl Res 62:609–617. https://doi.org/10.1007/s10344-016-1034-9
Robinson BG, Franke A, Derocher AE (2014) The influence of weather and lemmings on spatiotemporal variation in the abundance of multiple avian guilds in the Arctic. PLoS ONE 9:e101495. https://doi.org/10.1371/journal.pone.0101495
Rodríguez-Caro RC, Lima M, Anadón JD et al (2016) Density dependence, climate and fires determine population fluctuations of the spur-thighed tortoise Testudo graeca. J Zool 300:265–273. https://doi.org/10.1111/jzo.12379
Rondinini C, Battistoni A, Peronace V, Teofili C (eds) (2013) Lista Rossa IUCN dei Vertebrati Italiani. Comitato Italiano IUCN e Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Roma, IT
Sands JP, DeMaso SJ, Schnupp MJ, Brennan LA (2012) Wildlife science: connecting research with management. CRC Press, Boca Raton, FL
Scandura M, Iacolina L, Apollonio M et al (2010) Current status of the Sardinian partridge (Alectoris barbara) assessed by molecular markers. Eur J Wildlife Res 56:33–42. https://doi.org/10.1007/s10344-009-0286-z
Schielzeth H, Nakagawa S (2013) Nested by design: model fitting and interpretation in a mixed model era. Methods Ecol Evol 4:14–24. https://doi.org/10.1111/j.2041-210x.2012.00251.x
Sinclair ARE, Fryxell JM, Caughley G, Caughley G (2006) Wildlife ecology, conservation, and management, 2nd edn. Blackwell Publishing, Malden, MA, Oxford
Sutherland WJ, Newton I, Green R (2004) Bird ecology and conservation: a handbook of techniques. Oxford University Press, Oxford, New York
Thomas L, Buckland ST, Rexstad EA et al (2010) Distance software: design and analysis of distance sampling surveys for estimating population size. J Appl Ecol 47:5–14
Tian S, Xu J, Li J et al (2018) Research advances of Galliformes since 1990 and future prospects. Avian Res 9:32. https://doi.org/10.1186/s40657-018-0124-7
Walther BA, Chen JR-J, Lin H-S, Sun Y-H (2017) The effects of rainfall, temperature, and wind on a community of montane birds in Shei-Pa National Park. Taiwan Zool Stud 56:23
Wellendorf SD, Palmer WE, Bromley PT (2004) Estimating calling rates of Northern Bobwhite coveys and measuring abundance. J Wildlife Manage 68:672–682. https://doi.org/10.2193/0022-541X(2004)068[0672:ECRONB]2.0.CO;2
Zahran MA, Gilbert FS (2010) Climate—vegetation: Afro-Asian Mediterranean and Red Sea coastal lands. Springer, Dordrecht, New York
Zhao Y, Gong P, Yu L et al (2014) Towards a common validation sample set for global land-cover mapping. Int J Remote Sens 35:4795–4814. https://doi.org/10.1080/01431161.2014.930202
Zheng-Ji P, Yue-Hua S (1997) Reproductive success of hazel grouse at Changbai Mountain. Acta Zoologica Sinica 43:279–284
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer Science + Business Media, LLC, New York
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank Claudia Elisa Cinerari, Alice Draghi, Nicola Floris, Nicola Gilio, Marco Lombardini, Linda Mazzoleni, Maurizio Medda, Marco Murru, Ambra Repossi and Ugo Ziliani for the help in the fieldwork. We thank also the Regional Forest Agency for Land and Environment of Sardinia (Fo.Re.S.T.A.S., Agenzia Forestale Regionale per lo Sviluppo del Territorio e dell'Ambiente della Sardegna).
Funding
Open access funding provided by Università degli Studi di Pavia within the CRUI-CARE Agreement. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This research was conducted with ethical approval from the University of Pavia (Department of Earth and Environmental Sciences). Bird surveys were conducted with permission from local landowners where necessary.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiatante, G., Giordano, M., Vidus Rosin, A. et al. Spatial distribution of the Barbary Partridge (Alectoris barbara) in Sardinia explained by land use and climate. Eur J Wildl Res 67, 78 (2021). https://doi.org/10.1007/s10344-021-01519-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-021-01519-w