Abstract
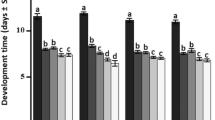
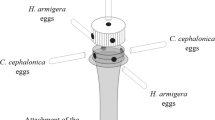
Trichogramma spp. are major parasitoids of lepidopteran pest eggs, but there is large variation in efficacy toward a given pest among the numerous described Trichogramma species. It is important to select the Trichogramma species that most effectively parasitize and develop in target pest eggs for biological control. In this context, Trichogramma pretiosum, T. exiguum, T. atopovirilia and T. acacioi were studied in Heliothis virescens eggs under different thermal conditions. The parasitoids were reared at constant temperatures of 20, 25 and 30°C and tested at these respective temperatures, while parasitoids reared at 25°C were also tested at 20 and 30°C, for a total of 20 species–temperature combinations. About 30 H. virescens eggs were offered to the parasitoids for 24 h. Among the four species, parasitism rate by T. atopovirilia was highest at all temperature conditions, whereas T. acacioi had the lowest rates of parasitism at 25°C and 25/30°C. Parasitism ranged from 13.8 to 43.8% among all species–temperature combinations. Viability (emerged parasitoids) ranged from 80.8 to 98.4%, and was deemed satisfactory. The emergence rates of T. exiguum and T. acacioi were affected by temperature. Temperature also affected the sex ratio of T. exiguum at 25/30°C, whereas T. pretiosum and T. acacioi produced females predominantly independent of temperature. Overall, the parasitoid T. atopovirilia was the most efficient in parasitizing H. virescens eggs, though the levels of parasitism obtained might not ensure its successful use in biological control programs. The temperature-related differences in biological traits observed in the four Trichogramma species tested hint at the importance of making careful choices regarding climatic conditions where the parasitoid is going to be used when selecting a species for biological control programs.


Similar content being viewed by others
References
Agamy E (2010) Field evaluation of the egg parasitoid, Trichogramma evanescens West. against the olive moth Prays oleae (Bern.) in Egypt. J Pest Sci 83:53–58
Andrade GS, Pratissoli D, Barros R, Zago HB, Pereira AID, Dalvi LP (2010) Screening of two Trichogramma species, native to southeastern Brazil, for the control of tobacco budworm. Rev Colomb Entomol 36:16–19
Bueno RCOF, Carneiro TR, Pratissoli D, Bueno AF, Fernandes OA (2008) Biology and thermal requirements of Telenomus remus reared on fall armyworm Spodoptera frugiperda eggs. Cienc Rural 38:1–6
Cônsoli FL, Parra JRP (1995) Effects of constant and alternating temperatures on Trichogramma galloi Zucchi (Hym., Trichogrammatidae) biology. Development and thermal requirements. J Appl Ent 119:415–418
Cook DR, Leonard BR, Gore J, Temple JH (2005) Baseline responses of bollworm, Helicoverpa zea (Boddie), and tobacco budworm. Heliothis virescens (F.), to indoxacarb and pyridalyl. J Agr Urban Entomol 22:99–109
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
Desneux N, Ramirez-Romero R (2009) Plant characteristics mediated by growing conditions can impact parasitoid’s ability to attack host aphids in winter canola. J Pest Sci 82:335–342
Desneux N, Wajnberg E, Fauvergue X, Privet S, Kaiser L (2004) Sublethal effects of a neurotoxic insecticide on the oviposition behaviour and the patch-time allocation in two aphid parasitoids, Diaeretiella rapae and Aphidius matricariae. Entomol Exp Appl 112:227–235
Desneux N, Fauvergue X, Dechaume-Moncharmont FX, Kerhoas L, Ballanger Y, Kaiser L (2005) Diaeretiella rapae limits Myzus persicae populations following applications of deltamethrin in oilseed rape. J Econ Entomol 98:9–17
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Desneux N, Barta RJ, Delebecque CJ, Heimpel GE (2009a) Transient host paralysis as a means of reducing self-superparasitism in koinobiont endoparasitoids. J Insect Physiol 55:321–327
Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009b) Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160:387–398
Desneux N, Star P, Delebecque CJ, Gariepy TD, Barta RJ, Hoelmer KA, Heimpel GE (2009c) Cryptic species of parasitoids attacking the soybean aphid, Aphis glycines Matsumura (Hemiptera: Aphididae), in Asia: Binodoxys communis Gahan and Binodoxyx koreanus Stary sp. n. (Hymenoptera: Braconidae: Aphidiinae). Ann Entomol Soc Am 102:925–936
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narváez-Vasquez CA, González-Cabrera J, Catalán Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbaneja A (2010) Biological invasion of European tomato crops by Tuta absoluta: Ecology, history of invasion and prospects for biological control. J Pest Sci 83:197–215
Emana GD (2007) Comparative studies of the influence of relative humidity and temperature on the longevity and fecundity of the parasitoid, Cotesia flavipes. J Insect Sci 7:1–9
Fernandes MG, Busoli AC, Degrande PE (1999) Parasitismo natural de ovos de Alabama argillacea Hüb. e Heliothis virescens Fab. (Lep.: Noctuidae) por Trichogramma pretiosum Riley (Hym.: Trichogrammatidae) em algodoeiros no Mato Grosso do Sul. An Soc Entomol Bras 28:695–701
Foerster MR, Foerster LA (2009) Effects of temperature on the immature development and emergence of five species of Trichogramma. Biocontrol 54:445–450
Gassmann AJ, Carriere Y, Tabashnik BE (2009) Fitness costs of insect resistance to Bacillus thuringiensis. Annu Rev Entomol 54:147–163
Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, Chichester
Greene GL, Leppla NC, Dickerson WA (1976) Velvetbean caterpillar: a rearing procedure and artificial medium. J Econ Entomol 69:487–488
Harrison WW, King EG, Ouzts JD (1985) Development of Trichogramma exiguum and T. pretiosum at five temperature regimes. Environ Entomol 14:118–121
Heckel DG, Gahan LJ, Daly JC, Trowell S (1998) A genomic approach to understanding Heliothis and Helicoverpa resistance to chemical and biological insecticides. Phil Trans R Soc B 353:1713–1722
Hoffmann AA, Hewa-Kapuge S (2000) Acclimation for heat resistance in Trichogramma nr. brassicae: can it occur without costs? Func Ecol 14:55–60
Hoffmeister TS, Roitberg BD (1997) To mark the host or the patch: decisions of a parasitoid searching for concealed host larvae. Evol Ecol 11:145–168
Hohmann CL, Santos WJ (1989) Parasitismo de ovos de Heliothis spp. e Alabama argillacea (Hübner) (Lepidoptera, Noctuidae) em algodoeiro por Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) no norte do Paraná. An Soc Entomol Bras 18:161–167
Hong SY, Jeong D, Gil HW, Yang JO, Lee EY, Hong SY (2009) The estimation of pesticide exposure in depression scores: in case of Korean orchard farmers. J Pest Sci 82:261–265
Klug T, Meyhöfer R (2009) Performance of two Trichogramma brassicae strains under greenhouse and field conditions for biocontrol of the silver Y moth in spinach cultures. J Pest Sci 82:73–79
Li LY (1994) Worldwide use of Trichogramma for biological control on different crops: a survey. In: Wajnberg E, Hassan S (eds) Biological control with egg parasitoids. Cab International, Oxon, pp 37–53
Maceda A, Hohmann L, Santos HR (2003) Temperature effects on Trichogramma pretiosum and Trichogrammatoidea annulata De Santis. Braz Arch Biol Technol 46:27–32
Mansour M (2010) Effects of gamma radiation on the Mediterranean flour moth, Ephestia kuehniella, eggs and acceptability of irradiated eggs by Trichogramma cacoeciae females. J Pest Sci 83:243–249
McCaffery AR (1998) Resistance to insecticides in heliothine lepidoptera: a global view. Phil Trans R Soc B 353:1735–1750
Melo RL, Pratissoli D, Polanczyk RA, Melo DF, Barros R, Milanez AM (2007) Biology and thermal requirements of Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae) parasitizing eggs of Diaphania hyalinata L. (Lepidoptera: Pyralidae). Neotrop Entomol 36:431–435
Mills N (2010) Egg parasitoids in biological control and integrated pest management. In: Consoli FL, Parra JRP, Zucchi RA (eds) Egg parasitoids in agroecosystems with emphasis on Trichogramma. Springer, Dordrecht, pp 389–411
Molina RMS, Fronza V, Parra JRP (2005) Seleção de Trichogramma spp., para o controle de Ecdytolopha aurantiana, com base na biologia e exigências térmicas. Rev Bras Entomol 49:152–158
Moraes CM, Consuelo M, Mescher MC (2005) Intrinsic competition between larval parasitoids with different degrees of host specificity. Ecol Entomol 30:564–570
Moscardini VF, Moura AP, Carvalho GA, Lasmar O (2008) Efeito residual de inseticidas sintéticos sobre Trichogramma pretiosum Riley (Hym., Trichogrammatidae) em diferentes gerações. Acta Sci Agron 30:177–182
Mulatu B, Applebaum SW, Coll M (2006) Effect of tomato leaf traits on the potato tuber moth and its predominant larval parasitoid: a mechanism for enemy-free space. Biol Control 37:231–236
Oliveira HN, Pratissoli D, Colombi CA, Polanczyk RA, Dalvi LP (2007) Tabela de vida de fertilidade de Trichogramma exiguum (Hymenoptera: Trichogrammatidae). Idesia 25:73–76
Pastori PL, Monteiro LB, Botton M, Pratissoli D (2007) Parasitism capacity of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) reared under different temperatures on Bonagota salubricola (Meyrick) (Lepidoptera: Tortricidae) eggs. Neotrop Entomol 36:926–931
Pennacchio F, Strand MR (2006) Evolution of developmental strategies in parasitic hymenoptera. Annu Rev Entomol 51:233–258
Pereira FF, Barros R, Pratissoli D (2004) Desempenho de Trichogramma pretiosum Riley e T. exiguum Pinto & Platner (Hymenoptera: Trichogrammatidae) submetidos a diferentes densidades de ovos de Plutella xylostella (Lepidoptera: Plutellidae). Cienc Rural 34:1669–1674
Pintureau B, Chapelle L, Delobel B (1999) Effects of repeated thermic and antibiotic treatments on a Trichogramma (Hym., Trichogrammatidae) symbiont. J Appl Entomol 123:473–483
Pintureau B, Pizzol J, Bolland P (2003) Effects of endosymbiotic Wolbachia on the diapause in Trichogramma hosts and effects of the diapause on Wolbachia. Entomol Exp Appl 106:193–200
Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J Pest Sci 83:447–452
Prasad RP, Roitberg BD, Henderson DE (2002) The effect of rearing temperature on parasitism by Trichogramma sibericum Sorokina at ambient temperatures. Biol Control 25:110–115
Pratissoli D, Fornazier MJ (1999) Ocorrência de Trichogramma acacioi Brun. Moraes & Soares (Hym.: Trichogrammatidae) em ovos de Nipteria panacea Thierry-Mieg (Lep.: Geometridae), um geometrídeo desfolhador do abacateiro. An Soc Entomol Bras 28:347–349
Pratissoli D, Oliveira HN (1999) Influência da idade dos ovos de Helicoverpa zea (Boddie) no parasitismo de Trichogramma pretiosum Riley. Pesq Agrop Bras 34:891–896
Pratissoli D, Vianna UR, Andrade JS, Pinon TBM, Andrade GS (2005) Thermal requirements of Trichogramma pretiosum and T. acacioi (Hym.: Trichogrammatidae), parasitoids of the avocado defoliator Nipteria panacea (Lep.: Geometridae), in eggs of two alternative hosts. Braz Arch Biol Technol 48:523–529
Pratissoli D, Polanczyk RA, Andrade GS, Holtz AM, Silva AF, Pastori PL (2007) Tabela de vida de fertilidade de cinco linhagens de Trichogramma pretiosum Riley (Hym.: Trichogrammatidae) criadas em ovos de Tuta absoluta (Merick) (Lep.: Gelechiidae), sob temperaturas constantes e alternadas. Cienc Rural 37:618–622
Ragsdale DW, Landis DA, Brodeur J, Heimpel GE, Desneux N (2011) Ecology and management of the soybean aphid in North America. Annu Rev Entomol 56:375–399
Ramalho FS, Azeredo TL, Fernandes FS, Nascimento Júnior JL, Malaquias JB, Nascimento ARB, Silva CAD, Zanuncio JC (2011) Food intake and utilization of Alabama argillacea (Hübner) (Lepidoptera: Noctuidae) fed on cotton cultivars with colored fibers. J Pest Sci. doi:10.1007/s10340-010-0341-2
Ratte HT (1985) Temperature and insect development. In: Hoffmann KH (ed) Environmental physiology and biochemistry of insects. Springer, Berlin, pp 33–66
Reznik SY, Voinovich ND, Vaghina NP (2009) Effect of temperature on the reproduction and development of Trichogramma buesi (Hymenoptera: Trichogrammatidae). Eur J Entomol 106:535–544
Rosenheim JA, Mangel M (1994) Patch-leaving rules for parasitoids with imperfect host discrimination. Ecol Entomol 19:374–380
Rousse P, Gourdon F, Roubaud M, Chiroleu F, Quilici S (2009) Biotic and abiotic factors affecting the flight activity of Fopius arisanus, an egg-pupal parasitoid of fruit fly pests. Environ Entomol 38:896–903
Saber M (2011) Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology. doi:10.1007/s10646-011-0704-3
Scott M, Berrigan D, Hoffmann AA (1997) Costs and benefits of acclimation to elevated temperature in Trichogramma carverae. Entomol Exp Appl 85:211–219
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Suckling DM, Brockerhoff EG (2010) Invasion biology, ecology, and management of the light brown apple moth (Tortricidae). Annu Rev Entomol 55:285–306
Tabone T, Bardon C, Desneux N, Wajnberg E (2010) Comparative assessment of parasitism of different Trichogramma spp.on Plutella xylostella L. on greenhouse cauliflower. J Pest Sci 83:251–256
van Alphen JJM, Visser ME (1990) Superparasitism as an adaptive strategy for insect parasitoids. Annu Rev Entomol 35:59–79
van Lenteren JC, Hale A, Klapwijk JN, van Schelt J, Steinberg S (2003) Guidelines for quality control of commercially produced natural enemies. In: van Lenteren JC (ed) Quality control and production of biological control agents: theory and testing procedures. CAB, Cambridge, England, pp 265–303
Vianna UR, Pratissoli D, Zanuncio JC, Lima ER, Brunner J, Pereira FF, Serrao JE (2009) Insecticide toxicity to Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) females and effect on descendant generation. Ecotoxicology 18:180–186
Acknowledgments
We thank Jeannine Pizzol, Jetske deBoer and Emily Mohl for their comments on the mansucript. This work was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior and in part by the Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Andrade, G.S., Pratissoli, D., Dalvi, L.P. et al. Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J Pest Sci 84, 313–320 (2011). https://doi.org/10.1007/s10340-011-0364-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-011-0364-3