Abstract
Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is a polyphagous pest with a wide geographic distribution. This pest first arrived in Brazil in 2013, and since then studies on possible control methods for it have been necessary. A possible method for the control of H. armigera is using the egg parasitoid Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Therefore, the objective of this study was to evaluate the performance of T. pretiosum on H. armigera eggs, which are known to represent suitable hosts for the development of this parasitoid species in the laboratory. Parasitism and emergence rates and the duration of the egg-to-adult period of T. pretiosum were investigated following 24- and 48-h exposures of this parasitoid to H. armigera and Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) eggs. The longevity of offspring after the 24-h exposure was studied, as well as the frequency of parasitism and emergence, host preference, and the emergence of offspring from eggs of different ages or oviposited by lepidopterans on different days. Parasitism was 14.4 and 34.9% more frequent on C. cephalonica than on H. armigera after 24 and 48 h of exposure, respectively. In C. cephalonica, parasitism was 27.2% higher after 48 h. Parasitism was more frequent on C. cephalonica eggs collected on the second day of oviposition (76.2%), and on H. armigera on the third day of oviposition (71.1%). Parasitism frequency was lower on 2-day-old C. cephalonica eggs (63.3%) and on 3-day-old H. armigera eggs (41.3%). When tested with a chance of choice between hosts, T. pretiosum preferred H. armigera, while in the test with no chance of choice there was no difference in preference. Thus, T. pretiosum may be considered a tool for the Integrated Pest Management (IPM) of H. armigera.
Similar content being viewed by others
Introduction
Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) is an important agricultural pest worldwide because it is highly polyphagous and damages several crops of economic importance, such as tomato, cotton, soybean, corn, and sunflower1,2,3,4. This species was first reported in Brazil in 2013 at high population sizes on soybean, corn, and cotton crops5,6. During its occurrence, farmers lost an estimated R$ 10 billion7.
The egg parasitoid Trichogramma spp. Westwood (Hymenoptera: Trichogrammatidae) is used in several countries for the control of lepidopteran pests8,9,10 by inundative application on millions of hectares of economically important agricultural crops8,9,11,12.
To meet demands for the biological control of pests of such crops as maize, soybean, cotton, tomato, and sugarcane some companies perform the mass-rearing of these parasitoids, and in Brazil four biofactories that produce Trichogramma spp. are registered in the Phytosanitary Agrochemicals System13. Among the species in the genus Trichogramma, T. pretiosum Riley (Hymenoptera: Trichogrammatidae) has been commercialized for the control of lepidopteran eggs13,14,15, and has been reported in approximately 18 different hosts and in 13 crops16.
In this context, T. pretiosum can be considered one of the tools available for use in the H. armigera Integrated Pest Management (IPM) program. However, to obtain good results using this parasitoid in the field, basic studies are necessary to better understand its development and behavior in the eggs of this pest. Therefore, the objective of the present study was to investigate the parasitism behavior of T. pretiosum on H. armigera eggs, compared with that on Corcyra cephalonica (Stainton) (Lepidoptera: Pyralidae) eggs, which are known to represent suitable hosts for the development of this parasitoid species in the laboratory.
Materials and Methods
Insects
Colonies of the three species of insects used in the experiments were kept in the laboratory in a room under controlled conditions at a temperature of 25 ± 1 °C, relative humidity (RH) of 70 ± 10%, and photophase of 12 h of light and 12 h of darkness. C. cephalonica eggs were obtained from Embrapa Soja of Londrina, PR, Brazil, and kept in the laboratory since 2014 following the methodology described by Bernardi et al.17. H. armigera individuals were obtained from soybean crops in Luís Eduardo Magalhães, BA, Brazil (12°5′58″S, 45°47′54″W), and were reared in the laboratory for five generations following the methodology described by Abbasi et al.18. T. pretiosum individuals were obtained from BUG Agentes Biologicos, Piracicaba, SP, Brazil, and reared in the laboratory following the methodology described by Parra19. The T. pretiosum colony was composed only of females, which reproduced by thelytokous parthenogenesis20.
Parasitism by T. pretiosum on H. armigera eggs
Eggs were used that were up to 24 h of age (i.e. up to 24 h after female oviposition), from the first day of female oviposition on C. cephalonica (alternative host) and H. armigera (natural host). The egg volume of H. armigera is 0.08 mm3 21, and that of C. cephalonica is 0.036 mm3 22. The eggs were glued to light blue paper (3.5 × 1.5 cm) with arabic gum (50%) diluted in deionized water. C. cephalonica eggs were handled with a soft-bristled brush, while those of H. armigera were cut out individually from the paper that was used as an oviposition substrate and glued to the light blue paper. Both types of eggs were exposed to germicidal light for 45 min to arrest their development. Each experimental replicate corresponded to a flat-bottomed glass tube (8.0 cm high × 2.0 cm in diameter), which contained a piece of light blue paper with eggs and a female parasitoid, and was covered with polyvinyl chloride plastic film. A droplet of honey was placed on the inner surfaces of the tubes to feed the females. Twenty replicates were used, with 30 eggs per host. Two sets of females were used in different treatments, one in which the female was allowed to parasitize the eggs for 24 h, while in the other treatment it was allowed to parasitize the eggs for 48 h, after which they were withdrawn and discarded. Female wasps used in the experiments were up to 24-h-old. The tubes were subsequently checked for adult emergence once per day. Parasitism frequency was evaluated by counting the number of dark eggs (signaling the occurrence of parasitism), and the emergence rate was determined by counting the number of dark eggs with holes. The duration of the period elapsed from the egg to adult parasitoid was also determined, and was measured from the day of parasitism to the day of offspring emergence. Laboratory conditions were controlled at 25 ± 2 °C with a 70 ± 10% RH and a 12-h photoperiod during these experiments.
Longevity of T. pretiosum
After the emergence of the parasitoids in response to the 24-h treatment described in the previous section (“Parasitism by Trichogramma on H. armigera eggs”), 40 females from both hosts were randomly selected and used in further experiments to determine the longevity of the adults. The females were kept individually in flat-bottomed glass tubes (8.0 cm high × 2.0 cm in diameter), which contained a honey droplet on their inner surface for food and were covered with polyvinyl chloride plastic film. Each wasp was provided honey ad libitum, and thus food level did not affect how long each wasp lived. Laboratory conditions were controlled at 25 ± 2 °C with a 70 ± 10% RH and a 12-h photoperiod.
Parasitism of T. pretiosum on eggs oviposited by H. armigera on different days
Eggs from the first, second, third, fourth, fifth, and sixth day of C. cephalonica and H. armigera oviposition were used in this test. Thirty eggs from each host collected on different days of oviposition were exposed to a T. pretiosum female for 24 h in a flat-bottomed glass tube (8.0 cm height × 2.0 cm in diameter) containing a droplet of honey on the inner-side surface and covered with polyvinyl chloride plastic film. The eggs used in the different treatments were at the same developmental stage (<24 h post-oviposition). The eggs were attached to light blue paper and placed under germicidal light for 45 min, as described in a previous section (“Parasitism by Trichogramma on H. armigera eggs”). Fifteen replicate tests were performed, with 30 eggs used for each host, in which parasitism and parasitoid emergence frequencies were evaluated. Female wasps used in the experiment were up to 24-h-old. Laboratory conditions were controlled at 25 ± 2 °C with a 70 ± 10% RH and a 12-h photoperiod.
Parasitism by T. pretiosum on eggs of H. armigera of different ages
Each female parasitoid was placed in a glass tube (8.0 cm height × 2.0 cm in diameter) covered with polyvinyl chloride plastic film and with a honey droplet provided on the inner wall for food. Thirty C. cephalonica eggs that were 1-, 2-, 3-, or 4-days-old were obtained from laboratory rearing, glued onto light blue paper (3.5 × 1.5 cm), and then placed into the glass tubes. This procedure was repeated with H. armigera eggs. The eggs used in this experiment were not placed under germicidal light because in this experiment we wanted to study the development of the embryo after parasitism. Female wasps used in the experiment were up to 24-h-old. After 24 h of parasitism, T. pretiosum females were removed and discarded. Fifteen replicates were observed, with 30 eggs for each host. The tubes containing light blue paper were kept in a heated room, under the conditions described previously, until the offspring emerged. The percentages of eggs that were parasitized and underwent parasitoid emergence were also evaluated. Laboratory conditions were controlled at 25 ± 2 °C with a 70 ± 10% RH and a 12-h photoperiod.
Host preference of T. pretiosum
Arenas were set up that were 4-cm high, made of transparent polyethylene acrylic, and contained four Duran tubes arranged equidistantly from the covering holes (Fig. 1)23,24. The insects could move between the different eggs in each tube to choose between them based on touch (egg-size measuring) and odor. In the test with a double chance of two choices, light blue pieces of paper (0.4 × 2.0 cm) containing 15 C. cephalonica eggs each were placed in two opposing tubes, and 15 H. armigera eggs were placed in the other two tubes. In the no-chance tests, only two tubes containing 15 eggs of either C. cephalonica or H. armigera were placed in the arena. The eggs were placed under germicidal light for 45 min before being used in the test. A female was released into each arena, through a hole located in the top part of the cap. After 24 h, the Duran tubes with the eggs were removed from the arena, covered with polyvinyl chloride plastic film, and kept in a heated room until the adults emerged. Fifteen replicates were observed for each treatment, and the frequencies of parasitism and emergence of offspring were evaluated. Laboratory conditions were controlled at 25 ± 2 °C with a 70 ± 10% RH and a 12-h photoperiod.
Data analyses
Data on the parasitism, emergence, and egg-to-adult period of T. pretiosum were submitted to the Kolmogorov and Bartlett tests to verify the normality of their residuals and the homogeneity of their variances, respectively. The data that met these assumptions were then submitted to analysis of variance (ANOVA). When there were two treatments, the Student’s t-test was used to compare different treatment conditions to each other, and when there were more than two treatments the Student-Newman-Keuls test was used (P < 0.05). When the data did not meet the requirements for ANOVA, the most adequate transformation was used, and if they still did not present normal residuals and homogeneous variances the data were then submitted to non-parametric tests. For the non-parametric tests, the Wilcoxon test was used to compare two treatments and the Kruskal–Wallis test was used to compare three or more treatments (p < 0.05). All analyses were conducted using the SAS software25.
Survival curves were also constructed using survival data at specific ages, and were compared according to the Kaplan-Meyer methodology26 and analyzed using SAS software25.
The frequency data from the choice tests were analyzed using Proc FREQ25 and interpreted by the chi-square (χ2) test, in which 1:1 was the null hypothesis assumed if the parasitoid had no preference for one host over the other.
Results
Parasitism by T. pretiosum on eggs of H. armigera
Comparing parasitism on the same host over time, an increase of 27.2% was observed when the female T. pretiosum was exposed to eggs of C. cephalonica for 48 h compared to that after 24 h (t = −4.90, df = 43, p < 0.0001). For H. armigera, similar levels of parasitism were observed after 24 and 48 h of exposure (t = −0.92, df = 43, p = 0.3603). When the hosts were compared within the same exposure periods, there was 14.4 and 34.9% greater percent parasitism on eggs of the alternative host C. cephalonica compared to that on H. armigera following 24 h (t = 2.69, df = 38, p = 0.0106) and 48 h (t = 5, df = 48, p < 0.0001) of parasitoid exposure, respectively (Table 1).
The duration of the egg-to-adult period of T. pretiosum was 10 days on both hosts after 24 h of egg exposure. At 48 h, the duration of the developmental period was longer on C. cephalonica eggs (11.3 days) than on H. armigera eggs (10.9 days) (z = 2.36, df = 1, p = 0.0182) (Table 2).
The emergence of parasitoids was not affected by the different hosts following 24 h of exposure (t = 0.82, df = 37, p = 0.4165). However, after 48 h of exposure percent emergence was 29.2% higher on C. cephalonica eggs than on H. armigera eggs (t = 5.62, df = 48, p < 0.0001). For both hosts, there was a significant difference in the percent emergence between exposure times. The emergence rates on C. cephalonica (t = 4.28, df = 43, p = 0.0001) and H. armigera eggs (t = 9.08, df = 43, p < 0.0001) were increased 1.2- and 1.86-fold after 24 h (Table 3).
Longevity of T. pretiosum
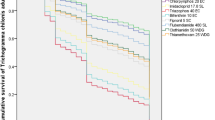
Following emergence in host eggs parasitized for 24 h, T. pretiosum females from C. cephalonica had a longevity of up to 12 days, which differed in relation to those from H. armigera, who had a longevity of up to 13 days. However, 3 days after emergence the percentage of surviving adults that had emerged from H. armigera eggs was reduced to 50%. The equivalent reduction was observed only on day 8 for insects that had emerged from C. cephalonica eggs. The survival of insects from C. cephalonica eggs was lower than that of those from H. armigera eggs on day 11 only (Fig. 2).
Parasitism of T. pretiosum on eggs obtained on different days of H. armigera oviposition
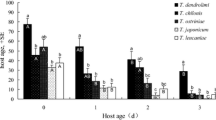
For C. cephalonica (χ2 = 11.89, df = 5, p = 0.0362), the parasitism frequency of T. pretiosum was similar for eggs obtained on different days of oviposition, with the exception of the first day, on which parasitism was 20.2% less frequent than that on the second day. On eggs of H. armigera (χ2 = 34.03, df = 5, p < 0.0001), the third day of oviposition presented the highest percent parasitism (71.1%) (Table 4).
On the first (t = 2.18, df = 28, p = 0.0375), second (t = 3.40, df = 28, p = 0.0021), fourth (t = 2.32, df = 28: p = 0.0281), and sixth (t = 4.50, df = 23, p = 0.0002) days of oviposition, the percent parasitism of C. cephalonica eggs was higher than that on H. armigera eggs (Table 4).
The percent emergence ranged from 88.7 to 97.6%, and was similar between oviposition periods for C. cephalonica (χ2 = 8.71, df = 5, p = 0.1211) and H. armigera (χ2 = 8.28, df = 5, p = 0.1414), as well as between these hosts.
Parasitism of T. pretiosum on eggs of H. armigera of different ages
The percent parasitism of T. pretiosum was lower on 2-day-old C. cephalonica eggs (χ2 = 16.30, df = 3, p = 0.0010, 63.3%) and 3-day-old H. armigera eggs (χ2 = 19.99, df = 3, p = 0.0002, 41.3%) than on host eggs of other ages (Table 5).
One- and 3-day-old eggs (t = 3.78, df = 28, p = 0.0008) presented 15 and 37.6% higher percent parasitism, respectively, on C. cephalonica than on H. armigera (Table 5).
The percent emergence was not influenced by the age of eggs in either C. cephalonica (χ2 = 0.07, df = 3, p = 0.9949) or H. armigera (χ2 = 3.25, df = 3, p = 0.3545), and ranged from 91.1 to 96.4%.
Host preference of T. pretiosum
In the test where the parasitoid was given a chance of choice between hosts (χ2 = 20.12, df = 1, p < 0.0001), T. pretiosum preferred to parasitize H. armigera eggs (80%) over those of C. cephalonica (20%). In the tests with no chance of choice (χ2 = 1.1843, df = 1, p = 0.2765), there was no preference of the parasitoid for one host over the other (Fig. 3).
There was no difference between the hosts in the emergence of adults observed; in the tests with a chance of choice (χ2 = 0.0179, df = 1, p = 0.8937) and no chance of choice (χ2 = 1.2678, df = 1, p = 0.2602), T. pretiosum grew equally well on both C. cephalonica and H. armigera eggs.
Discussion
The present study contributes basic information useful for studies aiming to achieve the management of an introduced pest in Brazil, H. armigera, using the egg parasitoid T. pretiosum, which is efficient at controlling some lepidopteran pests in several crops of economic importance16,27.
The parasitism frequency of T. pretiosum over 24 and 48 h of exposure to C. cephalonica and H. armigera eggs was higher in the alternative host, C. cephalonica, which is used to maintain laboratory rearing cultures of the parasitoid. The percent parasitism at 24 and 48 h on H. armigera eggs was lower than that reported by Ballal and Singh28, which was 76.7% under laboratory conditions when using an Indian population of the same pest. However, before tests were done the authors of the present study reared the parasitoid for two generations on H. armigera eggs. Therefore, it is possible that the better performance of the parasitoid on C. cephalonica eggs was related to behavioral adaptation (i.e. to insect conditioning), as it was maintained for successive generations in the laboratory on the eggs of this host29,30,31. In the laboratory, insects can adapt their behavior to the conditions in which they are maintained, both in adults and in the young29. However, previous results showed that the measures of laboratory performance used (fecundity and offspring sex ratio) were good predictors of field success in T. pretiosum32.
However, the parasitism frequency on H. armigera eggs observed herein was approximately 50% (49.7%), which suggests that the release of T. pretiosum in the field would need to be combined with other tools to control the pest effectively. T. pretiosum can be released in conjunction with the application of chemical and biological products, which must be efficient for use in the control of the pest but also selectively harmless to the parasitoid33,34. In sweetcorn crops in eastern Australia, Trichogramma release was conducted along with the use of bacteria- and virus-based biological products in IPM for the control of H. armigera35. In China, Trichogramma species are also used for pest management in maize, including the control of the eggs of H. armigera36.
In Turkey, the release of 120,000 Trichogramma parasitoids per hectare to control H. armigera on cotton crops resulted in parasitism frequency being reduced to 52.5% of its initial value37, highlighting the potential of Trichogramma for use in the biological control of H. armigera.
The percent emergence was similar between the hosts tested following 24 h of exposure, which suggests that the eggs of both species permit the complete development of the parasitoid38. However, with 48 h of exposure the emergence rate was reduced in both species to values below the ideal value of 85%10. It is possible that by remaining in contact with the eggs for a longer period, some females may deposit their eggs onto a host that is already parasitized, either by itself or by another female, which results in superparasitism. This behavior may impair the development of the parasitoid within the egg, or even kill both the developing parasitoid and the host. When a host is superparasitized, some or all of the immature parasitoids are insufficiently nourished, and thus fail to fully develop and die. In some cases, insects are born with a smaller size or with deformations39,40,41. Superparasitism may therefore reduce the parasitoid’s reproductive success42. Furthermore, in larger hosts gregarious development may occur, as is the case for H. armigera eggs and could explain the apparently reduced levels of parasitism on this species43.
In practice, our results regarding exposure time differences suggest that there is a need for periodic releases of the parasitoid, since the parasitoid will not maintain its population at the necessary level required in the field to control the pest once emergence has declined.
EMBRAPA (the Brazilian Agricultural Research Corporation) recommends that the parasitoid is released when, during sampling with adhesive pheromone traps, the first adults of H. armigera are collected44. However, in this study the highest frequencies of parasitism occurred on eggs collected on the third day of pest oviposition. These results are of great importance to the release of T. pretiosum as they will help the parasitoid to be released at the most appropriate time to best contribute to the efficient control of H. armigera in the field.
Regarding the ages of the parasitized eggs, there was reduced parasitism on 2-day-old C. cephalonica eggs and 3-day-old H. armigera eggs compared to that at other ages. The age of host eggs may influence the performance of egg parasitoids used in biological control45, since eggs may undergo morphological and physiological changes that may interfere with their acceptance by the female46,47. As the host egg is in a transitional stage of development, the parasitoid must kill the embryo and prevent its development, and then subsequently oviposit its eggs48.
In 3-day-old H. armigera eggs, it is possible that the embryo is already developing and occupying most of the total volume of the egg, with a high level of sclerotization, which increases the protection of the host and decreases the amount of food available for the development of a Trichogramma larva inside the egg48.
In general, it can be assumed that there was parasitism on H. armigera eggs of all ages, indicating the acceptance of the eggs of this species by the parasitoid, even at older stages. If the environmental conditions are favorable to the parasitoid insect for a longer period in the field, it may continue to parasitize pest eggs of all ages present on the crop, preventing the larvae from hatching.
The age of the egg following parasitism did not affect the emergence of the parasitoid offspring in this study, as was also previously observed with T. pretiosum on Mocis latipes eggs (Guenée) (Lepidoptera: Noctuidae)49, T. galloi Zucchi (Hymenoptera: Trichogrammatidae) on Diatraea saccharalis (Fabricius) (Lepidoptera: Crambidae)50, T. cacoeciae Marchal (Hymenoptera: Trichogrammatidae) on Lobesia botrana (Denis and Schiffermüler) (Lepidoptera: Tortricidae)12, and T. Chilonis Ishii (Hymenoptera: Trichogrammatidae) on Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae)51. Therefore, the offspring and their descendants should be able to establish persistent populations in the areas in which they are released.
In the preference tests conducted, when a chance of choice between host species was provided to the parasitoid, it preferred the eggs of the natural host, H. armigera. Several factors influence host preference, such as egg size, which is a critical factor in host selection. In general, most Trichogramma species tend to prefer to oviposit on medium-to large-sized eggs52. In the present study, the eggs of H. armigera and C. cephalonica had an approximate volume of 0.08 mm3 and 0.036 mm3, respectively21,22, which may be one of the factors explaining this preference. Further studies on the mechanisms associated with host selection should be performed with T. pretiosum and H. armigera to guarantee the success of the application of this parasitoid in the biological control of the pest.
The results obtained in this study demonstrated the possibility of using T. pretiosum in the control of H. armigera. Semi-field and field tests should be performed to adjust and optimize the release methodology, as well as studies of the association of this parasitoid with other pest control techniques.
References
Fitt, G. P. The ecology of Heliothis in relation to agroecosystems. Annu. Rev. Entomol. 34, 17–52 (1989).
Singh, S. P. Old World bollworm Helicoverpa armigera, associated Heliothinae and their natural enemies. Technical Bulletin, 135 p (2002).
Srivastava, C. P., Nitin, J. & Trivedi, T. P. Forecasting of Helicoverpa armigera populations and impact of climate change. Indian. J. Agric. Sci. 80, 3–10 (2010).
Cunningham, J. P. & Zalucki, M. P. Understanding Heliothine (Lepidoptera: Heliothinae) pests: what is a host plant? J. Econ. Entomol. 107, 881–96 (2014).
Czepak, C., Albernaz, K. C., Vivan, L. M., Guimarães, H. O. & Carvalhais, T. Primeiro registro de ocorrência de Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) no Brasil. Pesqui. Agropecu. Trop. 431, 110–113 (2013).
Tay, W. T. et al. A brave New World for an Old World pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. Plos one 8, e80134, https://doi.org/10.1371/journal.pone.0080134 (2013).
Gottems, L. Brasil já perdeu 10 bilhões com Helicoverpa armigera, http://www.agrolink.com.br/noticias/NoticiaDetalhe.aspx?codNoticia Accessed Dec05, 2017 (2013).
Smith, S. M. Biological control with Trichogramma: advances, success, and potential of their use. Annu. Ver. Entomol. 41, 375–406 (1996).
Van Lenteren, J. C. Commercial availability of biological control agents. In: van Lenteren, J. C. (ed.) Quality control and production of biological control agents: theory and testing procedures. CAB International, Wallingford, UK, pp 167–179 (2003).
Mills, N. Egg parasitoids in biological control and integrated pest management. In: Cônsoli F. L., Parra J. R. P., Zucchi R. A. (eds) Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Springer, Dordrecht, The Netherlands, pp 389–412 (2010).
Parra, J. R. P. & Zucchi, R. A. Trichogramma in Brazil: feasibility of use after twenty years of research. Neotrop. Entomol. 33, 271–281 (2004).
Pizzol, J., Desneux, N., Wajnberg, E. & Thiery, D. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J. Pest Sci. 85, 489–496 (2012).
Agrofit/Mapa Sistema de agrotóxicos fitossanitários. Coordenação Geral de Agrotóxicos e Afins, http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons Accessed Dec 05, 2016 (2016).
Parra, J. R. P. Mass rearing of egg parasitoids for biological control programs. In: Cônsoli, F. L., Parra, J. R. P., Zucchi, R. A. (eds) Egg parasitoids in agrosystems with emphasis on Trichogramma. Springer, New York, NY, USA, pp 267–292 (2010).
Parra, J. R. P. Biological control in Brazil: an overview. Sci. Agric. 71, 345–355 (2014).
Zucchi, R. A., Querino, R. B. & Monteiro, R. C. Diversity and hosts of Trichogramma in the new world, with emphasis in South America. In: Cônsoli, F. L., Parra, J. R. P. & Zucchi, R. A. (eds) Egg parasitoids in agrosystems with emphasis on Trichogramma. Springer, New York, NY, USA, pp 219–236 (2010).
Bernardi, E. B., Haddad, M. L. & Parra, J. R. P. Comparison of artificial diets for rearing Corcyra cephalonica (Stainton, 1865) (Lep., Pyralidae) for Trichogramma mass production. Rev. Bras. Biol. 60, 45–52 (2000).
Abbasi, B. et al. Rearing of cotton bollworm, Helicoverpa armigera, on a tapioca based artificial diet. J. Insect Sci. 7, 35 (2007).
Parra, J. R. P. Mass rearing of egg parasitoids for biological control programs, p. 267-292. Consoli, F. L., Parra, J. R. P., Zucchi, R. A. (Eds.). Eggs parasitoids in agrosystems with emphasis on Trichogramma. 465p. (2010).
Pinto, J. D. & Stouthamer, R. Systematics of the Trichogrammatidae with emphasis on Trichogramma, Biological Control with egg parasitoids (ed. by Wajnberg, E. & Hassan, S. A.). CAB International, Wallingford, UK, pp. 1–36 (1994).
Anantanawat, K. J., Glatz, R. & Keller, M. A. Effect of induced tolerance to Bt toxin on the egg size of Helicoverpa armigera and parasitism by Trichogramma pretiosum. Physiol. Entomol. 41, 267–273 (2016).
Cônsoli, F. L., Kitajima, E. W. & Parra, J. R. P. Ultrastructure of the natural and factitious host eggs of Trichogramma galloi Zucchi and Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae). Int. J. Insect Morphol. Embryol. 28, 211–229 (1999).
Thuler, R. T., Volpe, H. X. L., De Bortoli, S. A., Goulart, R. M. & Viana, C. L. T. P. Metodologia para avaliação de preferência hospedeira de parasitoides do gênero Trichogramma Westood. Bol. Sanid. Veg. 33, 333–340 (2007).
Queiroz, A. P., Taguti, E. A., Bueno, A. F., Grande, M. L. M. & Costa, C. O. Host preferences of Telenomus podisi (Hymenoptera: Scelionidae): parasitism on eggs of Dichelops malacanthus, Euchistus heros, and Podisus nigrispinus (Hemiptera: Pentatomidae). Neotrop. Entomol. 47, 543–552 (2018).
SAS Institute Inc. SAS/IML® 14.1 User’s Guide. Cary, NC: SAS Institute Inc (2015).
Kaplan, E. L. & Meyer, P. Nonparametric estimation from incomplete observations. J. Amer. Statist Assoc. 53, 457–481 (1958).
Bueno, R. C. O. F., Bueno, A. F., Parra, J. R. P., Vieira, S. S. & Oliveira, L. J. Biological characteristics and parasitism capacity of Trichogramma pretiosum Riley (Hymenoptera, Trichogrammatidae) on eggs of Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae). Rev. Bras. Entomol. 54, 322–327 (2010).
Ballal, C. R. & Singh, S. P. The Effectiveness of Trichogramma chilonis, Trichogramma pretiosum and Trichogramma brasiliense (Hymenoptera: Trichogrammatidae) as Parasitoids of Helicoverpa armigera (Lepidoptera: Noctuidae) on Sunflower (Helianthus annuus) and Redgram (Cajanus cajan). Biocontrol Sci. Technol. 13, 231–240 (2003).
Kaiser, L., Pham-Delegue, M. H. & Masson, C. Behavioural study of plasticity in host preferences of Trichogramma maidis (Hym.: Trichogrammatidae). Physiol. Entomol. 14, 53–60 (1989).
Hommay, G., Gertz, C., Kienlen, J. C., Pizzol, J. & Chavigny, P. Comparison between the control efficacy of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae) and two Trichogramma cacoeciae Marchal strains against grapevine moth (Lobesia botrana Den. & Schiff.), depending on their release density. Biocontrol Sci. Techn. 12, 569–581 (2002).
Kölliker-Ott, U. M., Bigler, F. & Hoffmann, A. A. Does mass rearing of field collected Trichogramma brassicae wasps influence acceptance of european corn borer eggs? Entomol. Exp. Appl. 109, 197–203 (2003).
Coelho, A. Jr., Rugman-Jones, P. F., Reigada, C., Stouthamer, R. & Parra, J. R. P. Laboratory performance predicts the success of field releases in inbred lines of the egg parasitoid Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Plos One 11, e0146153, https://doi.org/10.1371/journal.pone.0146153 (2016).
Takada, Y., Kawamura, S. & Takada, T. Effects of various insecticides on the development of the egg parasitoid Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae). J. Econ. Entomol. 94, 1340–1343 (2001).
Goulart, R. M., Volpe, H. X. L., Vacari, A. M., Thuler, R. T. & De Bortoli, S. A. Insecticide selectivity to two species of Trichogramma in three different hosts, as determined by IOBC/WPRS methodology. Pest Manag. Sci. 68, 240–244 (2012).
Scholz, B. C. G., Monsour, C. J. & Zalucki, M. P. An evaluation of selective Helicoverpa armigera control options in sweet corn. Australian J. Exp. Agric. 38, 601–607 (1998).
Wang, Z. Y., He, K. L., Zhang, F., Lu, X. & Babendreier, D. Mass rearing and release of Trichogramma for biological control of insect pests of corn in China. Biol. Control 68, 136–144 (2014).
Oztemiz, S. Natural parasitism rate of Trichogramma evanescens (Hymenoptera: Trichogrammatidae) and its release efficacy against the cotton bollworm, Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) in the Cukurova region, Turkey. Entomol. News 119, 19–33 (2008).
Schmidt, J. M. & Smith, J. J. The mechanism by which the parasitoid wasp Trichogramma minutum responds to host clusters. Entomol. Exp. Appl. 39, 287–294 (1985).
Salt, G. Experimental studies in insect parasitism. IV. The effect of superparasitism on populations of Trichogramma evanescens. J. Exp. Biol. 13, 363–375 (1936).
van Alphen, J. J. M. & Visser, M. E. Superparasitism as an adaptive strategy for insect parasitoids. Annu. Rev. Entomol. 35, 59–79 (1990).
Dorn, S. & Beckage, N. Superparasitism in gregarious hymenopteran parasitoids: ecological, behavioural and physiological perspectives. Physiol. Entomol. 32, 199–211 (2007).
Moreira, M. D., Santos, M. C., Besera, E. B., Torres, J. B. & Almeida, R. P. Parasitism and superparasitism of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on Sitotroga cerealella (Oliver) (Lepidoptera: Gelechiidae) eggs. Neotrop. Entomol. 38, 237–242 (2009).
Bai, B., Luck, R. F., Forster, L., Stephens, B. & Janssem, J. A. M. The effect of host size on quality attributes of the egg parasitoid. Trichogramma pretiosum. Entomol. Exp. Appl. 64, 37–48 (1992).
EMBRAPA Ações emergenciais propostas pela Embrapa para o manejo integrado de Helicoverpa spp. em áreas agrícolas. 19 p, https://www.embrapa.br/ALERTA-HELICOVERPA Accessed Dec 05, 2016 (2013).
Pak, G. A., Buis, H. C. E. M., Heck, I. C. C. & Hermans, M. L. G. Behavioural variations among strains of Trichogramma spp.: Host-age selection. Entomol. Exp. Appl. 40, 247–258 (1986).
Godin, C. & Boivin, G. Effects of host age on parasitism and progeny allocation in Trichogrammatidae. Entomol. Exp. Appl. 97, 149–160 (2000).
Colazza, S., Peri, E., Salerno, G. & Conti, E. Host searching by egg parasitoids: exploitation of host chemical cues. In: Cônsoli, F. L., Parra, J. R. P. & Zucchi, R. A. (eds) Egg parasitoids in agrosystems with emphasis on Trichogramma. Springer, New York, NY, USA, pp 97–147 (2010).
Jarjees, E. A. & Merritt, D. J. The effect of parasitization by Trichogramma australicum on Helicoverpa armigera host eggs and embryos. J. Invertebr. Pathol. 85, 1–8 (2004).
Stinguel, P., Carvalho, J. R., Pratissoli, D., Zuim, V. & Mardgan, L. Efeito da idade dos ovos de Mocis latipes (Lepidoptera, Noctuidae) sobre o parasitismo de Trichogramma pretiosum (Hymenoptera, Trichogrammatidae) com diferentes idades. Nucleus 10, 265–274 (2013).
Oliveira, H. N., Santana, D. R. S., Bellon, P. P. & Oliveira, F. C. Age influence of Diatraea saccharalis (Lepidoptera: Crambidae) on the parasitism by Trichogramma galloi (Hymenoptera: Trichogrammatidae). Interciência 39, 46–48 (2014).
Miura, K. & Kobayashi, M. Effects of host-egg age on the parasitism by Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae), an egg parasitoid of the diamondback moth. Appl. Entomol. Zool. 33, 219–222 (1998).
Schmidt, J. M. Host recognition and acceptance by Trichogramma. In: Wajnberg, E. & Hassan, S. A. (eds) Biological control with egg parasitoids. CAB International, Wallingford, UK, pp 113–135 (1994).
Acknowledgements
We would like to thank the Coordination for the Improvement of Higher Level Personnel (CAPES) for supporting the first author through a scholarship.
Author information
Authors and Affiliations
Contributions
V.L.L., A.M.V. and S.A.D.B. designed the experiment; V.L.L., D.G.R., N.A.S., G.C.I., B.G.D. and V.F.P.C. collected data; A.M.V. analyzed the data; V.L.L. wrote the manuscript; and A.M.V., R.C.S.V. and S.A.D.B. read, corrected, and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Laurentis, V.L., Ramalho, D.G., Santos, N.A. et al. Performance of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on eggs of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Sci Rep 9, 1156 (2019). https://doi.org/10.1038/s41598-018-37797-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-37797-9
- Springer Nature Limited