Abstract
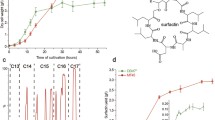
Bacillus velezensis B006 is a biocontrol agent which functions through effective colonization and surfactin production. To reveal the surfactin-producing mechanism, gas chromatography–mass spectrometry based untargeted metabolomics was performed to compare the metabolite profiles of strain B006 grown in industrial media M3 and M4. Based on the statistical and pathway topology analyses, a total of 31 metabolites with a fold change of less than − 1.0 were screened as the significantly altered metabolites, which distributed in 15 metabolic pathways. Fourteen amino acids involving in the metabolisms of alanine/aspartate/glutamate, glycine/serine/threonine, arginine/proline, glutathione/cysteine/methionine and valine/leucine/isoleucine as well as succinic acid in TCA cycle were identified to be the hub metabolites. Aminoacyl-tRNA biosynthesis, glycerolipid metabolism, and pantothenate/CoA biosynthesis also contributed to surfactin production. To the best of our knowledge, this study is the first to investigate the metabolic pathways of B. velezensis on surfactin production, and will benefit the optimization of commercial fermentation for higher surfactin yield.




Similar content being viewed by others
References
Abdel-Mawgoud AM, Aboulwafa MM, Hassouna NA (2008) Characterization of surfactin produced by Bacillus subtilis isolate BS5. Appl Biochem Biotechnol 150:289–303. https://doi.org/10.1007/s12010-008-8153-z
Berg J, Tymoczko JL, Stryer L (2006) Biochemistry, 6th edn. W. H Freeman, New York ISBN 0-7167-8724-5
Buchholz A, Hurlebaus J, Wandrey C, Takors R (2002) Metabolomics: quantification of intracellular metabolite dynamics. Biomol Eng 19:5–15. https://doi.org/10.1016/S1389-0344(02)00003-5
Cao P, Hu D, Zhang J, Zhang BQ, Gao Q (2017) Enhanced avermectin production by rational feeding strategies based on comparative metabolomics. Acta Microbiol Sin. 57:281–292 10.13343/j.cnki.wsxb.20160290
Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P (2014) Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol Plant Microb Interat 27:87–100. https://doi.org/10.1094/MPMI-09-13-0262-R
Cevallos-Cevallos JM (2013) Microbial metabolomics: towards pathogen detection and biological prospecting. Metabolomics 3:1. https://doi.org/10.4172/2153-0769.1000e125
Coutte F, Niehren J, Dhali D, John M, Versari C, Jacques P (2015) Modeling leucine’s metabolic pathway and knockout prediction improving the production of surfactin, a biosurfactant from Bacillus subtilis. Biotechnol J 10:1216–1234. https://doi.org/10.1002/biot.201400541
Deleu M, Lorent J, Lins L, Brasseur R, Braun N, EI Kirat K, Nylander T, Dufrêne YF, Mingeot-Leclercq MP (2013) Effects of surfactin on membrane models displaying lipid phase separation. Biochem Biophys Acta Biomembr 1828:801–815. https://doi.org/10.1016/j.bbamem.2012.11.007
Dhali D, Coutte F, Arias AA, Auger S, Bidnenko V, Chataigné G, Lalk M, Niehren J, de Sousa J, Versari C, Jacques P (2017) Genetic engineering of the branched fatty acid metabolic pathway of Bacillus subtilis for the overproduction of surfactin C14 isoform. Biotechnol J 12:1600574. https://doi.org/10.1002/biot.201600574
Ghribi D, Ellouze-Chaabouni S (2011) Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol Res Int 2011:653–654. https://doi.org/10.4061/2011/653654
Guo RJ, Wang JQ, Li SD, Jing YL, Gao YH, Sun RL (2018) A Bacillus velezensis strain and its application with mutifunctions in suppressing disease occurrence, promoting plant growth and drought resistance. Patent application number CH201810017581.8 (In chinese)
Gurjar J, Sengupta B (2015) Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresour Technol 189:243–249. https://doi.org/10.1016/j.biortech.2015.04.013
Haddad NI, Liu X, Yang S, Mu B (2008) Surfactin isoforms from Bacillus subtilis HSO121: separation and characterization. Protein Pept Lett 25:265–269. https://doi.org/10.2174/092986608783744225
Henkel M, Geissler M, Weggenmann F, Hausmann R (2017) Production of microbial biosurfactants: status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol J 12:1600561. https://doi.org/10.1002/biot.201600561
Henry G, Deleu M, Jourdan E, Thonart P, Ongena M (2011) The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell Microbiol 13:1824–1837. https://doi.org/10.1111/j.1462-5822.2011.01664.x
Ibba M, Francklyn C, Cusack S (2005) The aminoacyl-tRNA synthetases. Landes Bioscience, Georgetown (ISBN: 1587061899)
Jia K, Gao YH, Huang XQ, Guo RJ, Li SD (2015) Rhizosphere inhibition of cucumber fusarium wilt by different surfactin excreting strains of Bacillus subtilis. Plant Pathol J 31:140–151. https://doi.org/10.5423/PPJ.OA.10.2014.0113
Jia K, Li SD, Liu GJ, Guo RJ (2013) Characteristics of surfactin mutants of Bacillus subtilis B006 and their suppressing ability against cucumber Fusarium wilt. Chin J Biol Control 29:538–546
Jin H, Qiao F, Chen L, Lu C, Xu L, Gao X (2014) Serum metabolomic signatures of lymph node metastasis of esophageal squamous cell carcinoma. J Proteome Res 13:4091–4103. https://doi.org/10.1021/pr500483z
Jourdan E, Henry G, Duby F, Dommes J, Barthelemy JP, Thonart P, Ongena M (2009) Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol Plant Microb Interact 22:456–468. https://doi.org/10.1094/MPMI-22-4-0456
Kinsella K, Schulthess CP, Morris TF, Stuart JD (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379. https://doi.org/10.1016/j.soilbio.2008.11.019
Liu JF, Yang J, Yang SZ, Ye RQ, Mu BZ (2012) Effects of different amino acids in culture media on surfactin variants produced by Bacillus subtilis TD7. Appl Biochem Biotechnol 166:2091–2100. https://doi.org/10.1007/s12010-012-9636-5
Lu CZ, Yin J, Zhao FL, Li F, Lu WY (2017) Metabolomics analysis of the effect of dissolved oxygen on spinosad production by Saccharopolyspora spinosa. Antonie Van Leeuwenhoek 110:677–685. https://doi.org/10.1007/s10482-017-0835-5
Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M (2012) Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol 194:893–899. https://doi.org/10.1007/s00203-012-0823-0
Meyer H, Weidmann H, Lalk M (2013) Methodological approaches to help unravel the intracellular metabolome of Bacillus subtilis. Microb Cell Fact 12:69. https://doi.org/10.1186/1475-2859-12-69
Najmi Z, Ebrahimipour G, Franzetti A, Banat IM (2018) In situ downstream strategies for cost-effective bio/surfactant recovery. Biotechnol Appl Biochem. https://doi.org/10.1002/bab.1641
Nakano MM, Magnuson R, Myers A, Curry J, Grossman AD, Zuber P (1991) srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol 173:1770–1778
Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012) Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol 29:176–191. https://doi.org/10.1111/j.1574-6941.2011.01208.x
Ogura M, Yamaguchi H, Ki Yoshida, Fujita Y, Tanaka T (2001) DNA microarray analysis of Bacillus subtilis DegU, ComA and PhoP regulons: an approach to comprehensive analysis of B. subtilis two-component regulatory systems. Nucleic Acids Res 29:3804–3813. https://doi.org/10.1093/nar/29.18.3804
Ohno A, Ano T, Shoda M (1995) Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus subtilis in solid state fermentation. Biotechnol Bioeng 47:209–214
Ongena M, Jacques P (2008) Bacillus lipopeptides: versa-tile weapons for plant disease biocontrol. Trends Microbiol 16:115–125. https://doi.org/10.1016/j.tim.2007.12.009
Peypoux F, Michel G (1992) Controlled biosynthesis of Val7- and Leu7-surfactins. Appl Microbiol Biotechnol 36:515–517. https://doi.org/10.1007/BF00170194
Peyraud R, Kiefer P, Christen P, Massou S, Portais JC, Vorholt JA (2009) Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc Natl Acad Sci USA 06:4846–4851. https://doi.org/10.1073/pnas.0810932106
Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M (2010) Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. https://doi.org/10.1111/j.1574-6976.2010.00221.x
Sameh F, Krasimir D, Peggy V, Frédérique G, Guillaume D, Philippe J, Iordan N (2013) Oxygen transfer in three phase inverse fluidized bed bioreactor during biosurfactant production by Bacillus subtilis. Biochem Eng J 76:70–76. https://doi.org/10.1016/j.bej.2013.04.004
Serror P, Sonenshein AL (1996) CodY is required for nutritional repression of Bacillus subtilis genetic competence. J Bacteriol 178:5910–5915
Shi SB, Shen Z, Chen X, Chen T, Zhao XM (2009) Increased production of riboflavin by metabolic engineering of the purine pathway in Bacillus subtilis. Biochem Eng J 46:28–33. https://doi.org/10.1016/j.bej.2009.04.008
Slivinski CT, Mallmann E, de Araújo JM, Mitchell DA, Krieger N (2012) Production of surfactin by Bacillus pumilus UFPEDA 448 in solid-state fermentation using a medium based on okara with sugarcane bagasse as a bulking agent. Process Biochem 47:1848–1855
Strauch MA, Bobay BG, Cavanagh J, Yao F, Wilson A, Breton YL (2007) Abh and AbrB control of Bacillus subtilis antimicrobial gene expression. J Bacteriol 189:7720–7732. https://doi.org/10.1128/jb.01081-07
Wang JQ, Wang LG, Guo RJ, Ma GZ, Li SD (2017) Optimization of culture conditions for the enhancement of surfactin production from Bacillus substilis B006. Biotechnol Bull 33:214–221. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2017.04.028
Wei YH, Wang LF, Chang JS (2004) Optimizing iron supplement strategies for enhanced surfactin production with Bacillus subtilis. Biotechnol Prog 20:979–983
Willenbacher J, Rau JT, Rogalla J, Syldatk C, Hausmann R (2015) Foam-free production of surfactin via anaerobic fermentation of Bacillus subtilis DSM 10(T). AMB Express 5:21. https://doi.org/10.1186/s13568-015-0107-6
Willenbacher J, Yeremchuk W, Mohr T, Syldatk C, Hausmann R (2015) Enhancement of surfactin yield by improving the medium composition and fermentation process. AMB Express 5:145. https://doi.org/10.1186/s13568-015-0145-0
Wu QL (2007) Study on riboflavin fermentation optimization and metabolomics of recombinant Bacillus subtilis, Ph. D dissertation, Tianjin University
Xia ML, Huang D, Li SS, Wen JP, Jia XQ, Chen YL (2013) Enhanced FK506 production in Streptomyces tsukubaensis by rational feeding strategies based on comparative metabolic profiling analysis. Biotechnol Bioeng 110:2717–2730. https://doi.org/10.1002/bit.24941
Yang QY, Jia K, Geng WY, Guo RJ, Li SD (2014) Management of cucumber wilt disease by Bacillus subtilis B006 through suppression of Fusarium oxysporum f. sp. cucumerinum in rhizosphere. Plant Pathol J 13:160–166. https://doi.org/10.3923/ppj.2014.160.166
Yang QY, Suo YL, Guo RJ, Li SD, Xu XH (2012) Antifungal activities and principal component analysis of Bacillus subtilis B006 against Fusarium oxysporum f. sp. cucumerinum and Phytophthora capsici. Chin J Biol Control 8:235–242
Yao SL, Lu ZX, Hao TY, Lv FX, Bie XM (2014) Effect of amino acids and carbon backbone precursors on surfactin biosynthesis. J Nanjing Agric Univ 37:139–145. https://doi.org/10.7685/j.issn.1000-2030.2014.02.023
Yeh MS, Wei YH, Chang JS (2006) Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Biotechnol Progr 41:1799–1805. https://doi.org/10.1016/j.procbio.2006.03.027
Zhao PC, Quan CS, Jin LM, Wang LN, Wang JH, Fan SD (2013) Effects of critical medium components on the production of antifungal lipopeptides from Bacillus amyloliquefaciens Q-426 exhibiting excellent biosurfactant properties. World J Microbiol Biotechnol 29:401–409. https://doi.org/10.1007/s11274-012-1180-5
Zhi Y, Wu Q, Xu Y (2017) Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci Rep 7:40976. https://doi.org/10.1038/srep40976
Zhu LY, Liu Q, Liu Y, Li S (2015) Optimization of culture media for high production of surfactin C15 component by Bacillus subtilis. Chin J Bioprocess Eng 13:8–13
Zhu Z, Zhang FG, Wei Z, Ran W, Shen QR (2013) The usage of rice straw as a major substrate for the production of surfactin by Bacillus amyloliquefaciens XZ-173 in solid-state fermentation. J Environ Manag 127:96–102. https://doi.org/10.1016/j.jenvman.2013.04.017
Funding
This work was supported by the Program of China Agriculture Research System (CARS-23-D05), the Modern Agricultural Projects Funded by the Agriculture Department of Jiangsu Province (BE2016335), the Special Fund for Agri-Scientific Research in the Public Interest (201503112-2) and the Funds for Science and Technology Innovation Project from the Chinese Academy of Agricultural Sciences (CAAS-XTCX2016015).
Author information
Authors and Affiliations
Contributions
RG and SL as the corresponding authors conceived and supervised the study; JW, RG, and GM designed the experiments; JW and RG performed the experiments, analyzed the data and wrote the manuscript; and WW assisted on metabolomics experiments and data analysis. All authors approved the final article.
Corresponding authors
Ethics declarations
Participants
This article does not contain any studies with human participants.
Conflict of interest
The authors declare that they have no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Guo, R., Wang, W. et al. Insight into the surfactin production of Bacillus velezensis B006 through metabolomics analysis. J Ind Microbiol Biotechnol 45, 1033–1044 (2018). https://doi.org/10.1007/s10295-018-2076-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2076-7