Abstract
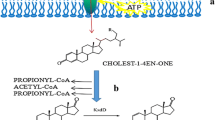
Androst-4-ene-3, 17-dione (AD) and androst-1, 4-diene-3, 17-dione (ADD) are generally produced by the biotransformation of phytosterols in Mycobacterium. The AD (D) production increases when the strain has high NAD+/NADH ratio. To enhance the AD (D) production in Mycobacterium neoaurum TCCC 11978 (MNR M3), a rational strategy was developed through overexpression of a gene involved in the phytosterol degradation pathway; NAD+ was generated as well. Proteomic analysis of MNR cultured with and without phytosterols showed that the steroid C27-monooxygenase (Cyp125-3), which performs sequential oxidations of the sterol side chain at the C27 position and has the oxidative cofactor of NAD+ generated, played an important role in the phytosterol biotransformation process of MNR M3. To improve the productivity of AD (D), the cyp125-3 gene was overexpressed in MNR M3. The specific activity of Cyp125-3 in the recombinant strain MNR M3C3 was improved by 22% than that in MNR M3. The NAD+/NADH ratio in MNR M3C3 was 131% higher than that in the parent strain. During phytosterol biotransformation, the conversion of sterols increased from 84 to 96%, and the yield of AD (D) by MNR M3C3 was increased by approximately 18% for 96 h fermentation. This rational strain modification strategy may also be applied to develop strains with important application values for efficient production of cofactor-dependent metabolites.






Similar content being viewed by others
References
Alfred W, Ansgar S (2006) Ethercarboxylic acid ester of sterol or stanol. US Patent No.US20060183723A1
Capyk JK, Kalscheuer R, Stewart GR, Liu J, Kwon H, Zhao R, Okamoto S, Jacobs WR, Eltis LD, Mohn WW (2009) Mycobacterial cytochrome p450 125 (Cyp125) catalyzes the terminal hydroxylation of C27 steroids. J Biol Chem 284(51):35534–35542
Chen YR, Huan HH, Cheng TF, Tang TY, Liu WH (2006) Expression of a cholesterol oxidase gene from Arthrobacter simplex in Escherichia coli and Pichia pastoris. Enzyme Microb Technol 39:258–262
Ding MZ, Cheng JS, Xiao WH, Qiao B, Yuan YJ (2009) Comparative metabolomic analysis on industrial continuous and batch ethanol fermentation processes by GC-TOF-MS. Metabolomics 5(2):229–238
García JL, Uhía I, Galán B (2012) Catabolism and biotechnological applications of cholesterol degrading bacteria. Microb Biotechnol 5(6):679–699
Garcia-Villalba R, Leon C, Dinelli G, Segura-Carretero A, Fernandez-Gutierrez A, Garcia-Canas V, Cifuentes A (2008) Comparative metabolomic study of transgenic versus conventional soybean using capillary electrophoresis-time-of-flight mass spectrometry. J Chromatogr A 1195(1–2):164–173
Geize RVD, Dijkhuizen L (2004) Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr Opin Microbiol 7:255–261
Geize RVD, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA 104:1947–1952
Ivashina TV, Nikolayeva VM, Dovbnya DV, Donova MV (2012) Cholesterol oxidase ChoD is not a critical enzyme accounting for oxidation of sterols to 3-keto-4-ene steroids in fast-growing Mycobacterium sp. VKM Ac-1815D. J Steroid Biochem 129:47–53
Li B, Wang W, Wang FQ, Wei DZ (2010) Cholesterol oxidase ChoL is a critical enzyme that catalyzes the conversion of diosgenin to 4-ene-3-keto steroids in Streptomyces virginiae IBL-14. Appl Microbiol Biotechnol 85:1831–1838
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔCT) method. Methods 25:402–408
Malaviya A, Gomes J (2008) Androstenedione production by biotransformation of phytosterols. Bioresour Technol 99:6725–6737
McLean KJ, Lafite P, Levy C, Cheesman MR, Mast N, Pikuleva IA, Leys D, Munro AW (2009) The structure of Mycobacterium tuberculosis CYP125: molecular basis for cholesterol binding in a P450 needed for host infection. J Biol Chem 284(51):35524–35533
Meyers PR, Bourn WR, Steyn LM, Helden PDV, Beyers AD, Brown GD (1998) Novel method for rapid measurement of growth of Mycobacteria in detergent-free media. J Clin Microbiol 36(9):2752–2754
Ouellet H, Guan S, Johnston JB, Chow ED, Kells PM, Burlingame AL, Cox JS, Podust LM, Ortiz de Montellano PR (2010) Mycobacterium tuberculosis CYP125A1, a steroid C27 monooxygenase that detoxifies intracellularly generated cholest-4-en-3-one. Mol Microbiol 77(3):730–742
Ouellet H, Johnston JB, de Montellano PR (2011) Cholesterol catabolism as a therapeutic target in Mycobacterium tuberculosis. Trends Microbiol 19(11):530–539
Perez C, Falero A, Duc HL, Balcinde Y, Hung BR (2006) A very efficient bioconversion of soybean phytosterols mixtures to androstanes by mycobacteria. J Ind Microbiol Biotechnol 33(8):719–723
Rosłoniec KZ, Wilbrink M, Moccia JK, Mohn WW, Geize RVD, Dijkhuizen L, Eltis LD (2009) Cytochrome P450 125 (CYP125) catalyses C26-hydroxylation to initiate sterol side-chain degradation in Rhodococcus jostii RHA1. Mol Microbiol 74(5):1031–1043
Shen YB, Wang M, Li HN, Wang YB, Luo JM (2012) Infuence of hydroxypropyl-β cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 39(9):1253–1259
Su LQ, Shen YB, Gao T, Luo JM, Wang M (2017) Improvement of AD biosynthesis response to enhanced oxygen transfer by oxygen vectors in Mycobacterium neoaurum TCCC 11979. Appl Biochem Biotechnol 182(4):1564–1574
Su LQ, Shen YB, Zhang WK, Gao T, Shang ZH, Wang M (2017) Cofactor engineering to regulate NAD+/NADH ratio with its application to phytosterols biotransformation. Microb Cell Fact 16(1):182–192
Szentirmai A (1990) Microbial physiology of side chain degradation of sterols. J Ind Microbiol Biotechnol 6(2):101–115
Uhía I, Galán B, Morales V, García JL (2011) Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2 155. Environ Microbiol 13(4):943–959
Wang J, Liu HH, Huang D, Jin LN, Wang C, Wen JP (2017) Comparative proteomic and metabolomic analysis of Streptomyces tsukubaensis reveals the metabolic mechanism of FK506 overproduction by feeding soybean oil. Appl Microbiol Biotechnol 101:2447–2465
Wei W, Fan SY, Wang FQ, Wei DZ (2014) Accumulation of androstadiene-dione by overexpression of heterologous 3-ketosteroid Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01. World J Microbiol Biotechnol 30:1947–1954
Wei W, Wang FQ, Fan SY, Wei DZ (2010) Inactivation and augmentation of the primary 3-ketosteroid-delta-1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microb 76(13):4578–4582
Yao K, Wang FQ, Zhang HC, Wei DZ (2013) Identifcation and engineering of cholesterol oxidases involved in the initial step of sterols catabolism in Mycobacterium neoaurum. Metab Eng 15:75–87
Yao K, Xu LQ, Wang FQ, Wei DZ (2014) Characterization and engineering of 3-ketosteroid-delta-1-dehydrogenase and 3-ketosteroid-9α-hydroxylase in Mycobacterium neoaurum ATCC 25795 to produce 9α-hydroxy-4- androstene-3, 17-dione through the catabolism of sterols. Metab Eng 24:181–191
Yang X, Dubnau E, Smith I, Sampson NS (2007) Rv1106c from Mycobacterium tuberculosis is a 3β-hydroxysteroid dehydrogenase. Biochemistry 46(31):9058–9067
Zhang YP, Huang ZH, Du CY, Li Y, Cao ZA (2009) Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab Eng 11:101–106
Acknowledgements
The authors would like to thank the members of the Industrial Fermentation Microbiology Laboratory in the Tianjin University of Science and Technology for their valuable comments and helpful discussions. This work was supported by the Key Project of Chinese Ministry of Education (213004A); the National Natural Science Foundation of China (21276196 and 21406167); Tianjin Municipal Science and Technology Commission (17PTGCCX00190); and Tianjin Programs for Science and Technology Development (15ZCZDSY00510).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Su, L., Shen, Y., Xia, M. et al. Overexpression of cytochrome p450 125 in Mycobacterium: a rational strategy in the promotion of phytosterol biotransformation. J Ind Microbiol Biotechnol 45, 857–867 (2018). https://doi.org/10.1007/s10295-018-2063-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2063-z