Abstract
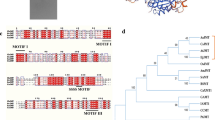
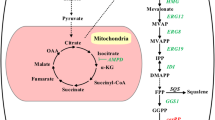
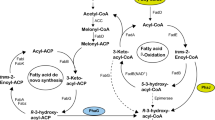
Thioesterases (TEs) play an essential role in the metabolism of fatty acids (FAs). To explore the role of TEs in mediating intracellular lipid metabolism in the oleaginous fungus Mortierella alpina, the acyl-CoA thioesterase ACOT8I was overexpressed. The contents of total fatty acids (TFAs) were the same in the recombinant strains as in the wild-type M. alpina, whilst the production of free fatty acids (FFAs) was enhanced from about 0.9% (wild-type) to 2.8% (recombinant), a roughly threefold increase. Linoleic acid content in FFA form constituted about 9% of the TFAs in the FFA fraction in the recombinant strains but only about 1.3% in the wild-type M. alpina. The gamma-linolenic acid and arachidonic acid contents in FFA form accounted for about 4 and 25%, respectively, of the TFAs in the FFA fraction in the recombinant strains, whilst neither of them in FFA form were detected in the wild-type M. alpina. Overexpression of the TE ACOT8I in the oleaginous fungus M. alpina reinforced the flux from acyl-CoAs to FFAs, improved the production of FFAs and tailored the FA profiles of the lipid species.









Similar content being viewed by others
References
Ando A, Sumida Y, Negoro H, Suroto DA, Ogawa J, Sakuradani E, Shimizu S (2009) Establishment of Agrobacterium tumefaciens-mediated transformation of an oleaginous fungus, Mortierella alpina 1S-4, and its application for eicosapentaenoic acid producer breeding. Appl Environ Microb 75(17):5529–5535. https://doi.org/10.1128/AEM.00648-09
Cantu DC, Chen YF, Reilly PJ (2010) Thioesterases: a new perspective based on their primary and tertiary structures. Protein Sci 19(7):1281–1295. https://doi.org/10.1002/pro.417
Chen LW, Zhang JH, Lee J, Chen WN (2014) Enhancement of free fatty acid production in Saccharomyces cerevisiae by control of fatty acyl-CoA metabolism. Appl Microbiol Biotechnol 98(15):6739–6750. https://doi.org/10.1007/s00253-014-5758-8
Chen HQ, Hao GF, Wang L, Wang HC, Gu Z, Liu LM, Zhang H, Chen W, Chen YQ (2015) Identification of a critical determinant that enables efficient fatty acid synthesis in oleaginous fungi. Sci Rep 5:11247. https://doi.org/10.1038/srep11247
Howard TP, Middelhaufe S, Moore K, Edner C, Kolak DM, Taylor GN, Parker DA, Lee R, Smirnoff N, Aves SJ et al (2013) Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in Escherichia coli. Proc Natl Acad Sci USA 110(19):7636–7641. https://doi.org/10.1073/pnas.1215966110
Hunt MC, Alexson SHE (2008) Novel functions of acyl-CoA thioesterases and acyltransferases as auxiliary enzymes in peroxisomal lipid metabolism. Prog Lipid Res 47(6):405–421. https://doi.org/10.1016/j.plipres.2008.05.001
Hunt MC, Siponen MI (1822) Alexson SEH (2012) The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim Biophys Acta Mol Basis Dis 9:1397–1410. https://doi.org/10.1016/j.bbadis.2012.03.009
Hao DH, Chen HQ, Hao GF, Yang B, Zhang BX, Zhang H, Chen W, Chen YQ (2015) Production of conjugated linoleic acid by heterologous expression of linoleic acid isomerase in oleaginous fungus Mortierella alpina. Biotechnol Lett 37(10):1983–1992. https://doi.org/10.1007/s10529-015-1871-8
Hao G, Chen H, Wang L, Gu Z, Song Y, Zhang H, Chen W, Chen YQ (2014) Role of malic enzyme during fatty acid synthesis in the oleaginous fungus Mortierella alpina. Appl Environ Microb 80(9):2672–2678. https://doi.org/10.1128/AEM.00140-14
Huang L, Zhao L, Zan X, Song Y, Ratledge C (2016) Boosting fatty acid synthesis in Rhodococcus opacus PD630 by overexpression of autologous thioesterases. Biotechnol Lett 38(6):999–1008. https://doi.org/10.1007/s10529-016-2072-9
Ishizuka M, Toyama Y, Watanabe H, Fujiki Y, Takeuchi A, Yamasaki S, Yuasa S, Miyazaki M, Nakajima N, Taki S et al (2004) Overexpression of human acyl-CoA thioesterase upregulates peroxisome blogenesis. Exp Cell Res 297(1):127–141. https://doi.org/10.1016/j.yexcr.2004.02.029
Jing FY, Cantu DC, Tvaruzkova J, Chipman JP, Nikolau BJ, Yandeau-Nelson MD, Reilly PJ (2011) Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem 12(1):44. https://doi.org/10.1186/1471-2091-12-44
Lennen RM, Pfleger BF (2012) Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol 30(12):659–667. https://doi.org/10.1016/j.tibtech.2012.09.006
Lu XF, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10(6):333–339. https://doi.org/10.1016/j.ymben.2008.08.006
Nie ZK, Ji XJ, Shang JS, Zhang AH, Ren LJ, Huang H (2014) Arachidonic acid-rich oil production by Mortierella alpina with different gas distributors. Bioprocess Biosyst Eng 37(6):1127–1132. https://doi.org/10.1007/s00449-013-1104-2
Rutter CD, Zhang SY, Rao CV (2015) Engineering Yarrowia lipolytica for production of medium-chain fatty acids. Appl Microbiol Biot 99(17):7359–7368. https://doi.org/10.1007/s00253-015-6764-1
Scharnewski M, Pongdontri P, Mora G, Hoppert M, Fulda M (2008) Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J 275(11):2765–2778. https://doi.org/10.1111/j.1742-4658.2008.06417.x
Tilton GB, Shockey JM, Browse J (2004) Biochemical and molecular characterization of ACH2, an acyl-CoA thioesterase from Arabidopsis thaliana. J Biol Chem 279(9):7487–7494. https://doi.org/10.1074/jbc.M309532200
Tillander V, Arvidsson Nordström E, Reilly J, Strozyk M, Van Veldhoven PP, Hunt MC, Alexson SE (2014) Acyl-CoA thioesterase 9 (ACOT9) in mouse may provide a novel link between fatty acid and amino acid metabolism in mitochondria. Cell Mol Life Sci 71(5):933–948. https://doi.org/10.1111/j.1365-2362.2005.01447.x
Wang L, Chen W, Feng Y, Ren Y, Gu ZN, Chen HQ, Wang HC, Thomas MJ, Zhang BX, Berquin IM (2011) Genome characterization of the oleaginous fungus Mortierella alpina. PLoS One 6(12):e28319. https://doi.org/10.1371/journal.pone.0028319
Zheng YN, Li LL, Liu Q, Yang JM, Wang XW, Liu W, Xu X, Liu H, Zhao G, Xian M (2012) Optimization of fatty alcohol biosynthesis pathway for selectively enhanced production of C12/14 and C16/18 fatty alcohols in engineered Escherichia coli. Microb Cell Fact 11:65. https://doi.org/10.1186/1475-2859-11-65
Author information
Authors and Affiliations
Contributions
JG carried out the experiments and drafted the manuscript. HC and BY performed the complementation experiments. HC and YQC analyzed the data and helped to draft the manuscript. WC and HZ conceived and coordinated the study and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This research was supported by the National Natural Science Foundation of China (nos. 31571810, 31530056); the Fundamental Research Funds for the Central Universities (no. JUSRP51702A); the program of “Collaborative innovation center of food safety and quality control in Jiangsu Province”.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Guo, J., Chen, H., Yang, B. et al. The role of acyl-CoA thioesterase ACOT8I in mediating intracellular lipid metabolism in oleaginous fungus Mortierella alpina. J Ind Microbiol Biotechnol 45, 281–291 (2018). https://doi.org/10.1007/s10295-018-2006-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-018-2006-8