Abstract
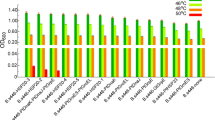
The response of Clostridium tyrobutyricum to butyric acid stress involves various stress-related genes, and therefore overexpression of stress-related genes can improve butyric acid tolerance and yield. Class I heat shock proteins (HSPs) play an important role in the process of protecting bacteria from sudden changes of extracellular stress by assisting protein folding correctly. The results of quantitative real-time PCR indicated that the Class I HSGs grpE, dnaK, dnaJ, groEL, groES, and htpG were significantly upregulated under butyric acid stress, especially the dnaK and groE operons. Overexpression of groESL and htpG could significantly improve the tolerance of C. tyrobutyricum to butyric acid, while overexpression of dnaK and dnaJ showed negative effects on butyric acid tolerance. Acid production was also significantly promoted by increased GroESL expression levels; the final butyric acid and acetic acid concentrations were 28.2 and 38% higher for C. tyrobutyricum ATCC 25755/groESL than for the wild-type strain. In addition, when fed-batch fermentation was carried out using cell immobilization in a fibrous-bed bioreactor, the butyric acid yield produced by C. tyrobutyricum ATCC 25755/groESL reached 52.2 g/L, much higher than that for the control. The improved butyric acid yield is probably attributable to the high GroES and GroEL levels, which can stabilize the biosynthetic machinery of C. tyrobutyricum under extracellular butyric acid stress.





Similar content being viewed by others
References
Andersch W, Bahl H, Gottschalk G (1983) Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol 18(6):327–332. doi:10.1007/bf00504740
Berterame NM, Porro D, Ami D, Branduardi P (2016) Protein aggregation and membrane lipid modifications under lactic acid stress in wild type and OPI1 deleted Saccharomyces cerevisiae strains. Microb Cell Fact 15:12. doi:10.1186/s12934-016-0438-2
Blum P, Ory J, Bauernfeind J, Krska J (1992) Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J Bacteriol 174(22):7436–7444
Buckel W, Dorn U, Semmler R (1981) Glutaconate CoA-transferase from Acidaminococcus fermentans. Eur J Biochem 118(2):315–321
Chen D-H, Madan D, Weaver J, Lin Z, Schr€oder GF, Chiu W, Rye HS (2013) Visualizing GroEL/ES in the act of encapsulating a folding protein. Cell 153(6):1354–1365. doi:10.1016/j.cell.2013.04.052
Chen YY, Stabryla L, Wei N (2016) Improved Acetic Acid Resistance in Saccharomyces cerevisiae by Overexpression of the WHI2 gene identified through inverse metabolic engineering. Appl Environ Microbiol 82(7):2156–2166. doi:10.1128/aem.03718-15
Consalvi V, Chiaraluce R (2001) Chaperonins eLS. Wiley, Newyork
Desmond C, Fitzgerald GF, Stanton C, Ross RP (2004) Improved stress tolerance of GroESL-overproducing Lactococcus lactis and orobiotic Lactobacillus paracasei NFBC 338. Appl Environ Microbiol 70(10):5929–5936. doi:10.1128/aem.70.10.5929-5936.2004
Guan NZ, Li JH, Shin HD, Du GC, Chen J, Liu L (2016) Metabolic engineering of acid resistance elements to improve acid resistance and propionic acid production of Propionibacterium jensenii. Biotechnol Bioeng 113(6):1294–1304. doi:10.1002/bit.25902
Hasunuma T, Sakamoto T, Kondo A (2016) Inverse metabolic engineering based on transient acclimation of yeast improves acid-containing xylose fermentation and tolerance to formic and acetic acids. Appl Microbiol Biotechnol 100(2):1027–1038. doi:10.1007/s00253-015-7094-z
Heap JT, Pennington OJ, Cartman ST, Minton NP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Methods 78(1):79–85. doi:10.1016/j.mimet.2009.05.004
Herrero AA, Gomez RF, Snedecor B, Tolman CJ, Roberts MF (1985) Growth inhibition of Clostridium thermocellum by carboxylic acids: a mechanism based on uncoupling by weak acids. Appl Microbiol Biotechnol 22(1):53–62. doi:10.1007/BF00252157
Horwich AL, Farr GW, Fenton WA (2006) GroEL-GroES-mediated protein folding. Chem Rev 106(5):1917–1930. doi:10.1021/cr040435v
Horwich AL, Low KB, Fenton WA, Hirshfield IN, Furtak K (1993) Folding in vivo of bacterial cytoplasmic proteins: role of GroEL. Cell 74(5):909–917. doi:10.1016/0092-8674(93)90470-B
Huang YL, Wu ZT, Zhang LK, Cheung CM, Yang ST (2002) Production of carboxylic acids from hydrolyzed corn meal by immobilized cell fermentation in a fibrous-bed bioreactor. Bioresour Technol 82(1):51–59. doi:10.1016/s0960-8524(01)00151-1
Jang YS, Im JA, Choi SY, Lee JI, Lee SY (2014) Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab Eng 23:165–174. doi:10.1016/j.ymben.2014.03.004
Jang YS, Woo HM, Im JA, Kim IH, Lee SY (2013) Metabolic engineering of Clostridium acetobutylicum for enhanced production of butyric acid. Appl Microbiol Biotechnol 97(21):9355–9363. doi:10.1007/s00253-013-5161-x
Jiang L, Cai J, Wang JF, Liang SZ, Xu ZN, Yang ST (2010) Phosphoenolpyruvate-dependent phosphorylation of sucrose by Clostridium tyrobutyricum ZJU 8235: Evidence for the phosphotransferase transport system. Bioresour Technol 101(1):304–309. doi:10.1016/j.biortech.2009.08.024
Jiang L, Cui HY, Zhu LY, Hu Y, Xu X, Li S, Huang H (2015) Enhanced propionic acid production from whey lactose with immobilized Propionibacterium acidipropionici and the role of trehalose synthesis in acid tolerance. Green Chem 17(1):250–259. doi:10.1039/c4gc01256a
Jiang L, Wang JF, Liang SZ, Cai J, Xu ZN (2011) Control and optimization of Clostridium tyrobutyricum ATCC 25755 adhesion into fibrous matrix in a fibrous bed bioreactor. Appl Biochem Biotechnol 165(1):98–108. doi:10.1007/s12010-011-9236-9
Jiang L, Wang JF, Liang SZ, Cai J, Xu ZN, Cen PL, Yang ST, Li SA (2011) Enhanced butyric acid tolerance and bioproduction by Clostridium tyrobutyricum immobilized in a fibrous bed bioreactor. Biotechnol Bioeng 108(1):31–40. doi:10.1002/bit.22927
Jiang L, Wang JF, Liang SZ, Wang XN, Cen PL, Xu ZN (2010) Production of butyric acid from glucose and xylose with immobilized cells of Clostridium tyrobutyricum in a fibrous-bed bioreactor. Appl Biochem Biotechnol 160(2):350–359. doi:10.1007/s12010-008-8305-1
Jiang L, Wang JF, Liang SZ, Wang XN, Cen PL, Xu ZN (2009) Butyric acid fermentation in a fibrous bed bioreactor with immobilized Clostridium tyrobutyricum from cane molasses. Bioresour Technol 100(13):3403–3409. doi:10.1016/j.biortech.2009.02.032
Kerner MJ, Naylor DJ, Ishihama Y, Maier T, Chang HC, Stines AP, Georgopoulos C, Frishman D, Hayer-Hartl M, Mann M, Hartl FU (2005) Proteome-wide analysis of chaperonin-dependent protein folding in Escherichia coli. Cell 122(2):209–220. doi:10.1016/j.cell.2005.05.028
Koch B, Kilstrup M, Vogensen FK, Hammer K (1998) Induced levels of heat shock proteins in a dnaK mutant of Lactococcus lactis. J Bacteriol 180(15):3873–3881
Lee J, Jang YS, Han MJ, Kim JY, Lee SY (2016) Deciphering Clostridium tyrobutyricum metabolism based on the whole-genome sequence and proteome analyses. mBio. doi:10.1128/mBio.00743-16
Lee S, Lee JH, Mitchell RJ (2015) Analysis of Clostridium beijerinckii NCIMB 8052’s transcriptional response to ferulic acid and its application to enhance the strain tolerance. Biotechnol Biofuels 8:14. doi:10.1186/s13068-015-0252-9
Liu X, Zhu Y, Yang ST (2006) Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol Prog 22(5):1265–1275. doi:10.1021/bp060082g
Liu XG, Yang ST (2006) Kinetics of butyric acid fermentation of glucose and xylose by Clostridium tyrobutyricum wild type and mutant. Process Biochem 41(4):801–808. doi:10.1016/j.procbio.2005.10.009
Malaviya A, Jang YS, Lee SY (2012) Continuous butanol production with reduced byproducts formation from glycerol by a hyper producing mutant of Clostridium pasteurianum. Appl Microbiol Biotechnol 93(4):1485–1494. doi:10.1007/s00253-011-3629-0
Mogk A, Homuth G, Scholz C, Kim L, Schmid FX, Schumann W (1997) The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. Embo J 16(15):4579–4590. doi:10.1093/emboj/16.15.4579
Papagianni M, Avramidis N (2011) Lactococcus lactis as a cell factory: A twofold increase in phosphofructokinase activity results in a proportional increase in specific rates of glucose uptake and lactate formation. Enzyme Microb Technol 49(2):197–202. doi:10.1016/j.enzmictec.2011.05.002
Parsons LM, Limberger RJ, Shayegani M (1997) Alterations in levels of DnaK and GroEL result in diminished survival and adherence of stressed Haemophilus ducreyi. Infect Immun 65(6):2413–2419
Rephaeli A, Zhuk R, Nudelman A (2000) Prodrugs of butyric acid from bench to bedside: Synthetic design, mechanisms of action, and clinical applications. Drug Dev Res 50(3–4):379–391
Rose IA (1955) Acetate kinase of bacteria (acetokinase): Acetate + ATP ⇌ Acetyl-P + ADP methods in enzymology. Academic Press, NewYork
Schumann W, Homuth G, Mogk A (1998) The GroE chaperonin machine is the major modulator of the CIRCE heat shock regulon of Bacillus subtilis. J Biosci 23(4):415–422. doi:10.1007/bf02936135
Selby K, Lindstrom M, Somervuo P, Heap JT, Minton NP, Korkeala H (2011) Important role of class i heat shock genes hrcA and dnaK in the heat shock response and the response to pH and NaCl stress of group I Clostridium botulinum strain ATCC 3502. Appl Environ Microbiol 77(9):2823–2830. doi:10.1128/aem.02633-10
Sillers R, Chow A, Tracy B, Papoutsakis ET (2008) Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 10(6):321–332. doi:10.1016/j.ymben.2008.07.005
Silva EM, Yang S-T (1995) Kinetics and stability of a fibrous-bed bioreactor for continuous production of lactic acid from unsupplemented acid whey. J Biotechnol 41(1):59–70. doi:10.1016/0168-1656(95)00059-Y
Tashiro Y, Shinto H, Hayashi M, Baba SI, Kobayashi G, Sonomoto K (2007) Novel high-efficient butanol production from butyrate by non-growing Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13 5 64) with methyl viologen. J Biosci Bioeng 104(3):238–240. doi:10.1263/jbb.104.238
Tomas CA, Welker NE, Papoutsakis ET (2003) Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell’s transcriptional program. Appl Environ Microbiol 69(8):4951–4965. doi:10.1128/aem.69.8.4951-4965.2003
Upadhyaya BP, DeVeaux LC, Christopher LP (2014) Metabolic engineering as a tool for enhanced lactic acid production. Trends Biotechnol 32(12):637–644
Wang QH, Venkataramanan KP, Huang HZ, Papoutsakis ET, Wu CH (2013) Transcription factors and genetic circuits orchestrating the complex, multilayered response of Clostridium acetobutylicum to butanol and butyrate stress. BMC Syst Biol 7:17. doi:10.1186/1752-0509-7-120
Williams DR, Young DI, Young M (1990) Conjugative plasmid transfer from Escherichia coli to Clostridium acetobutylicum. J General Microbiol 136(5):819–826. doi:10.1099/00221287-136-5-819
Wu ZT, Yang ST (2003) Extractive fermentation for butyric acid production from glucose by Clostridium tyrobutyricum. Biotechnol Bioeng 82(1):93–102. doi:10.1002/bit.10542
Yu MR, Du YM, Jiang WY, Chang WL, Yang ST, Tang IC (2012) Effects of different replicons in conjugative plasmids on transformation efficiency, plasmid stability, gene expression and n-butanol biosynthesis in Clostridium tyrobutyricum. Appl Microbiol Biotechnol 93(2):881–889. doi:10.1007/s00253-011-3736-y
Yu MR, Zhang YL, Tang IC, Yang ST (2011) Metabolic engineering of Clostridium tyrobutyricum for n-butanol production. Metab Eng 13(4):373–382. doi:10.1016/j.ymben.2011.04.002
Zhang CH, Yang H, Yang FX, Ma YJ (2009) Current progress on butyric acid production by fermentation. Curr Microbiol 59(6):656–663. doi:10.1007/s00284-009-9491-y
Zhang YL, Yu MR, Yang ST (2012) Effects of ptb knockout on butyric acid fermentation by Clostridium tyrobutyricum. Biotechnol Prog 28(1):52–59. doi:10.1002/btpr.730
Zhu Y, Liu XG, Yang ST (2005) Construction and characterization of pta gene-deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid fermentation. Biotechnol Bioeng 90(2):154–166. doi:10.1002/bit.20354
Zhu Y, Wu ZT, Yang ST (2002) Butyric acid production from acid hydrolysate of corn fibre by Clostridium tyrobutyricum in a fibrous-bed bioreactor. Process Biochem 38(5):657–666. doi:10.1016/s0032-9592(02)00162-0
Zhu Y, Yang ST (2004) Effect of pH on metabolic pathway shift in fermentation of xylose by Clostridium tyrobutyricum. J Biotechnol 110(2):143–157. doi:10.1016/j.jbiotec.2004.02.006
Zhu Y, Yang ST (2003) Adaptation of Clostridium tyrobutyricum for enhanced tolerance to butyric acid in a fibrous-bed bioreactor. Biotechnol Prog 19(2):365–372. doi:10.1021/bp025647x
Zingaro KA, Papoutsakis ET (2013) GroESL overexpression imparts Escherichia coli tolerance to i-, n-, and 2-butanol, 1,2,4-butanetriol and ethanol with complex and unpredictable patterns. Metab Eng 15:196–205. doi:10.1016/j.ymben.2012.07.009
Acknowledgements
This work was supported by the Natural Science Foundation of China (21276093) and the Planned Science and Technology Project of Guangdong Province, China (2015A050502014).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suo, Y., Luo, S., Zhang, Y. et al. Enhanced butyric acid tolerance and production by Class I heat shock protein-overproducing Clostridium tyrobutyricum ATCC 25755. J Ind Microbiol Biotechnol 44, 1145–1156 (2017). https://doi.org/10.1007/s10295-017-1939-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-017-1939-7